Abstract
Previous work has shown that integrin α1-null Alport mice exhibit attenuated glomerular disease with decreased matrix accumulation and live much longer than strain-matched Alport mice. However, the mechanism underlying this observation is unknown. Here we show that glomerular gelatinase expression, specifically matrix metalloproteinase-2 (MMP-2), MMP-9, and MMP-14, was significantly elevated in both integrin α1-null mice and integrin α1-null Alport mice relative to wild-type mice; however, only MMP-9 was elevated in glomeruli of Alport mice that express integrin α1. Similarly, cultured mesangial cells from α1-null mice showed elevated expression levels of all three MMPs, whereas mesangial cells from Alport mice show elevated expression levels of only MMP-9. In both glomeruli and cultured mesangial cells isolated from integrin α1-null mice, activation of the p38 and ERK branches of the mitogen-activated protein kinase pathway was also observed. The use of small molecule inhibitors demonstrated that the activation of the p38, but not ERK, pathway was linked to elevated MMP-2, -9, and -14 expression levels in mesangial cells from integrin α1-null mice. In contrast, elevated MMP-9 levels in mesangial cells from Alport mice were linked to ERK pathway activation. Blockade of gelatinase activity using a small molecule inhibitor (BAY-12-9566) ameliorated progression of proteinuria and restored the architecture of the glomerular basement membrane in α1 integrin-null Alport mice, suggesting that elevated gelatinase activity exacerbates glomerular disease progression in these mice.
The accumulation of extracellular matrix in the glomerular basement membrane (GBM) and the mesangium as a function of renal disease progression is a feature shared by a variety of glomerular diseases. This is true for Alport syndrome, in which defects in genes encoding basement membrane collagen α3(IV), α4(IV), or α5(IV) chains result in a delayed onset, progressive glomerulonephritis characterized by mesangial matrix expansion and GBM irregularities.1 A number of animal models for Alport syndrome exist, and most resemble human Alport syndrome with regard to renal disease.2 In earlier work, we showed that autosomal Alport [α3(IV) knockout] mice that are also null for α1β1 integrin (double knockout, or DKO, mice) display attenuated glomerular and tubulointerstitial pathogenesis and lived nearly twice as long as strain-matched Alport mice.3 These DKO animals showed reduced mesangial expansion and improved GBM architecture. The mechanism underlying the influence of α1 integrin on the progression of glomerular pathogenesis in these mice has not been elucidated.
Integrin α1β1 is expressed at high levels on glomerular mesangial cells.4 As a collagen-binding integrin, α1β1 has been implicated to function in mesangial cell adhesion, migration, and proliferation.5,6 α1β1 integrin appears to play a direct role in matrix remodeling, because neutralizing antibodies against either integrin subunit prevents collagen gel contraction by cultured rat mesangial cells.7,8 This effect is mediated via activation of the ERK 1/2 branch of the mitogen-activated protein kinase (MAPK) signal transduction pathway.9 These data are in contrast to genetic studies of both fibroblasts and vascular endothelial cells, which showed elevated matrix metalloproteinase (MMP) expression in α1 integrin-null cells versus wild-type cells.10,11,12 The effect of the α1-null genotype on mesangial cell expression of MMPs has not been explored.
Dysregulation of MMPs in glomerular mesangial cells has been implicated as contributing to the pathobiological mechanism for a number of glomerular diseases.13 Because glomerular disease is often associated with altered signaling of the MAPK pathway in mesangial cells,14,15 and because integrin α1β1-blocking studies of rat mesangial cells alter both collagen matrix remodeling and MAPK signaling,9 we surmised that the absence of α1 integrin might alter pathways that regulate matrix metabolism, and that this might help explain the reduced accumulation of glomerular matrix in the DKO mice. Here we show that the expression of MMP-2, MMP-9, and MMP-14 (also called MT-1 MMP) are significantly elevated in both glomeruli and cultured mesangial cells from integrin α1-null mice compared to wild-type mice, whereas only MMP-9 is up-regulated in Alport glomeruli and mesangial cells. Elevated expression can be abrogated in α1 integrin-null mesangial cells by blocking the activation of the p38, but not the ERK1/2 branch of MAPK. Conversely, the same strategy shows ERK regulation of MMP-9 in mesangial cells from Alport mice. Blocking the activity of these MMPs using a small molecule inhibitor attenuates progression of albuminuria in DKO mice, suggesting that overexpression of MMPs likely exacerbates disease progression in DKO mice. Thus, even though the net effect of the α1-null background in Alport mice is significant attenuation of glomerular disease,3 some metabolic changes (in this case, elevated gelatinase expression) occur that are actually deleterious to glomerular structure/function.
Materials and Methods
Administration of MMP Inhibitor to Alport Mice
The Alport, integrin α1-null, and DKO mice are all on the 129 Sv background and have been described previously.3,16,17 Wild-type mice (controls) are normal for both collagen α3(IV) alleles and are a product of double-heterozygote crosses for the Alport mutation, and thus also 129 Sv. The use of animals in this study was performed in accordance with an approved institutional animal care and use committee protocol. Extreme care was taken to minimize pain and discomfort. MMP inhibitor was administered between 4 and 7 weeks of age. BAY 12-9566 was emulsified in suspension with 0.5% carboxymethyl cellulose in water. Four mg were given once a day by oral gavage. The drug was freshly prepared before administration.
Glomerular Isolation
Isolation of mouse glomeruli was performed as described previously.18 Anesthetized mice are perfused transcardially with deactivated 4.5 μmol/L Dynabeads (Dynal Biotech, Oslo, Sweden). Kidneys are minced and digested with collagenase, and the glomeruli are isolated using a specialized magnet. We found these preparations to be consistently >97% pure, allowing reliable assessment of glomerular-specific mRNA and protein expression in mice.
Derivation and Qualification of Primary Mesangial Cell Cultures
The pelleted glomeruli were resuspended in 5 ml of 0.1% trypsin and 0.05% collagenase I (Worthington, Lakewood, NJ) in phosphate-buffered saline (PBS),19 and digested in the prewarmed solution for 2 hours at 37°C with moderate shaking. The glomeruli were disassociated, the suspension transferred to a fresh tube containing 10 ml of media plus fetal calf serum and spun at 1500 rpm for 10 minutes at 4°C. The pellet was resuspended in 12.5 ml of Dulbecco’s modified Eagle’s medium/Ham’s F-12 containing 20% fetal calf serum, penicillin, streptomycin, gentamycin, and glutamine, and then plated on a 10-cm tissue culture dish.20 The culture was left undisturbed for several days. Cells were grown to near confluency, trypsinized, and passed 1:3 in growth media. Within 24 hours the culture media was replaced with d-valine-containing minimal essential medium (Sigma, St. Louis, MO) completed as before. Cells were grown to near confluency, qualified (see below), and used. All cell culture reagents used were obtained from Gibco-Invitrogen Cell Culture (Carlsbad, CA) unless otherwise noted.
The decapsulated glomeruli used to initiate the culture contained only visceral epithelial cells. These cells are terminally differentiated and thus do not proliferate in culture. Epithelial contamination is therefore unlikely. The proliferation of the cells in d-valine containing media prevented fibroblast contamination of the culture.21 The cultures were very homogeneous in appearance after d-valine treatment. The cells displayed stellate, spindle-shaped morphology, accompanied by hills and valleys when confluent, which are all characteristics of mesangial cells.22 Features of epithelial and endothelial morphology were lacking.
Cells were qualified by immunostaining for desmin (Abcam, Cambridgeshire, UK), fibronectin (Sigma), smooth muscle actin (Sigma), and vimentin (Chemicon, Temecula, CA). All of which are major requisites to assign mesangial origin to the cells.22 Staining for von Willebrand factor protein (Santa Cruz Biotechnology, Santa Cruz, CA), an endothelial cell marker, was negative.
Flow Cytometry Analysis
Monoclonal antibodies against integrin α1β1 heterodimer and isotype controls were of hamster origin and described previously.5 Cultured integrin α1 +/+ or −/− murine mesangial cells were blocked in mouse sera 2% and resuspended in PBS containing 2% fetal calf serum. All antibody dilutions and washes were performed with this same buffer. The cells were stained with hamster antibodies to integrin α1 (Ha31/8, dilution 1:400; Biogen, Cambridge, MA) or an isotype-matched nonreactive control antibody (Ha4/8; dilution, 1:400) and washed three times. Cells were then reacted with an Alexa 488-conjugated goat anti-hamster secondary antibody (Molecular Probes, Eugene, OR) at 1:200 dilution, washed three times, and then fixed with 1% formalin. Flow cytometry data were acquired and analyzed using a FACS Aria and CellQuest Pro software (BD Biosciences, San Jose, CA).
MAPK Inhibitor Studies in Cultured Mesangial Cells
Mesangial cell cultures from wild-type, α1 integrin null DKO mice, and Alport mice were grown to 80% confluency each on 10-cm tissue culture dishes. Serum-containing media was replaced by serum-free media and incubated overnight (14 hours). Two dishes for each culture were then trypsinized and the cells pelleted. The cells were resuspended in serum-free media and plated on four collagen I (BD Biosciences)-coated 6-cm tissue culture dishes. The cells were allowed to attach overnight and confluence noted 14 hours later.
Stocks of the inhibitors herbimycin, SB203580, and PD98059 (Calbiochem, San Diego, CA) were prepared in anhydrous dimethyl sulfoxide. After a 1:100 dilution in serum-free media the inhibitors were placed on the cells. A final concentration of 0.5 μm of herbimycin, 10 μm of SB203580, and 40 μm of PD98059 was used versus dimethyl sulfoxide alone. Cells were incubated with inhibitors and dimethyl sulfoxide alone for 24 hours before harvesting.
Real-Time and Standard Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
RT-PCR and real-time PCR analysis for MMP-2, MMP-9, and MMP-14 and RT-PCR analysis for MMP-12 was performed as described previously.23,24 For TIMP amplifications, oligonucleotide primer pairs were as follows: for GAPDH, 5′-GGTGAAGGTCGGAGTCAACGGATTTGGTCG-3′ and 5′-GGATCTCGCTCCTGGAAGATGGTGATGGG-3′ (236-bp target size); for TIMP-1, 5′-GCATCTGGCATCCTCTTGTTG-3′ and 5′-GACAGTGTTCAGGCTTCAGTTTTTC-3′ (637-bp target size); for TIMP-2, 5′-CGTAGTGATCAGAGCCAAAGCA-3′and 5′-GGCTCTTCTTCTGGGTGATGCT-3′ (304-bp target size); for TIMP-3, 5′-CTGGCTTGGGCTTGTCGTGCTCCT-3′ and 5′-TGTGGCGTTGCTGATGCTCTTGTC-3′; for TIMP-4, 5′-TGTGGCGTTGCTGATGCTCTTGTC-3′ and 5′-CCAGCAGCCAGTCCGTCCAGA-3′ (269-bp target size) based on the published sequences. PCR was performed for 30 cycles for TIMP-1, -2, -3, and -4 and 27 cycles for GAPDH at annealing temperature of 60°C. Amplified products were separated on 2% agarose gel as described earlier.
Western Blot Analysis
Either isolated glomeruli or mesangial cells were lysed in RIPA buffer (radioimmunoprecipitation assay lysis buffer) (0.1% sodium dodecyl sulfate, 0.5% deoxycholate, 1% Nonidet P-40, 100 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4) containing a protease inhibitor cocktail (P8340, Sigma), 0.5 mmol/L dithiothreitol, and 0.5% phenylmethyl sulfonyl fluoride. Equal quantities of total protein (5 to 10 μg) was fractionated on polyacrylamide gel electrophoresis gels and transferred to nylon membranes. All antibodies were purchased from Cell Signaling Technologies (Danvers, MA), and all were derived from rabbit. The catalog numbers of anti-ERK and anti-pERK (Thr202/Tyr204) are 9102 and 9106s, respectively. Both antibodies were used at 1:1000 dilution. The catalog numbers of anti-p38 and pp38(Thr180/Tyr182) are 9212 and 9211s, respectively. Anti-p38 antibodies were diluted at 1:1000, and anti-pp38 antibodies were used at 1:500 dilution. Anti-ERK/p38 antibodies were diluted in 5% milk and anti-phosphorylated ERK/p38 antibodies were diluted in 3% bovine serum albumin. All experiments were repeated at least three times with independently derived cell cultures/glomeruli with nearly identical outcomes.
Immunohistochemistry
Cryosections (4 μm) of kidneys from 7-week-old wild-type and Alport mice were air-dried, fixed by immersion in ice cold acetone, and subjected to immunohistochemical staining analysis. Antibodies used were specific for fibronectin (rabbit polyclonal against human plasma fibronectin, used at 1:200; Sigma); rabbit anti-mouse MMP-2 (Chemicon) 1:100; rabbit anti-mouse MMP-9 (Chemicon) 1:100. Fluorescein isothiocyanate-conjugated anti-VLA1 antibodies were a gift from Philip Gotwals (Biogen Corp.) and used at 1:200 dilution. The laminin α2 chain-specific antibody was an anti-mouse rat monoclonal antibody purchased from Sigma-Aldrich (St. Louis, MO). All antibodies were diluted into 7% nonfat dry milk in PBS to reduce nonspecific binding. Primary antibodies were allowed to react for 2 hours at room temperature in a humidified chamber. After three 5-minute washes in PBS, slides were incubated with fluorescein isothiocyanate-conjugated secondary antibodies for 1 hour at room temperature (goat anti-rabbit, used at 1:200; Vector Laboratories, Burlingame, CA). The sections were coverslipped, sealed, and imaged. Images were collected using a Spot RT digital camera interfaced with an Olympus BH-2 fluorescence microscope (Olympus, Center Valley, PA).
Gelatin Zymography
Equal numbers of primary mesangial cells were plated on rat tail collagen matrix and cultured for 24 hours in serum-free media. The media was collected and substrate gel electrophoresis (zymography) was performed as described previously.25,26 Media conditioned by culturing the human fibrosarcoma cell line, HT1080, was used as a positive control. Zymography was performed using media from three different independently derived culture sets with qualitatively similar results.
Proteinuria
Urine was collected at weekly intervals starting when the animals were 4 weeks of age and ending at 7 weeks of age. Samples were analyzed for albumin and normalized to urinary creatinine using the QuantiChrom BCG albumin assay kit (DIAG-250) and the creatinine assay kit (DICT-500) according to the manufacturer’s instructions (BioAssay Systems, Hayward, CA). Samples from four to five mice per group were run in triplicate, and the mean values for each measurement plotted.
Transmission Electron Microscopy
Transmission electron microscopy was performed as described previously.3 All samples were from 7-week-old treated (BAY 12-9566 from 4 to 7 weeks of age) or untreated animals. At least six glomeruli were analyzed from three different animals for each treatment group, and representative transmission electron microscopy images are shown.
Data Presentation and Statistical Analysis
Data are expressed as mean ± SD. Differences between means were tested for significance using Student’s t-test. Differences were considered significant at the level of P < 0.05.
Results
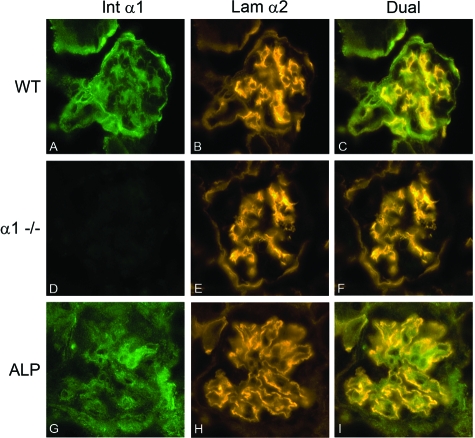
We previously reported that progressive glomerular pathology in the Alport mouse is characterized by a marked expansion of the mesangial matrix, and that Alport mice that are also null for α1 integrin do not show mesangial matrix expansion.3 Figure 1 demonstrates that α1β1 integrin is primarily expressed on mesangial cells in wild-type and Alport glomeruli, and is completely absent in glomeruli from integrin α1-null mice. Laminin α2 chain-specific immunostaining was used as a marker for mesangial matrix (Figure 1, B and E). As previously reported,3 laminin α2 localizes to both the mesangium and the GBM in Alport glomeruli (Figure 1H). Given that α1β1 integrin localizes to glomerular mesangium, we surmised that the α1 integrin influence on glomerular pathogenesis must emanate from the mesangial cell compartment.
Figure 1.
Integrin α1β1 is expressed on mesangial cells of wild-type and Alport glomeruli, and absent on mesangial cells from integrin α1-null mice. Glomeruli from 7-week-old wild-type (A–C), integrin α1-null (D–F), and Alport mice (G–I) showing immunostaining for integrin α1 (A, D, G), laminin α2 (B, E, and H), or dual immunostaining for both integrin α1 and laminin α2 (C, F, and I). In wild-type mice integrin α1 co-localizes with the mesangial cell marker laminin α2. In Alport glomeruli, the previously documented GBM localization of laminin α23 is contrasted with mesangial localization of integrin α1. Original magnifications, ×400.
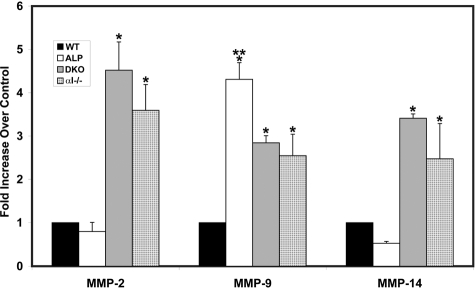
Because attenuated mesangial expansion in α1 integrin-null Alport mice might reflect altered matrix remodeling in integrin α1-null mesangial cells, we profiled expression of the gelatinases in glomeruli isolated from these mice. Glomeruli from 7-week-old wild-type, Alport, integrin α1-null, and DKO mice were collected using a magnetic bead isolation technique.18 RNA from four independent sets of mice was isolated and analyzed in triplicate experiments using real-time RT-PCR for expression of MMP-2, MMP-9, and MT1-MMP. As shown in Figure 2, expression of MMP-9 mRNA is significantly elevated (more than fourfold) in Alport glomeruli compared to wild-type controls. Expression of MMP-2 and MMP-14 mRNAs, however, did not vary significantly in glomeruli from wild-type versus Alport mice. In contrast, mRNAs for MMP-2, MMP-9, and MMP-14 were significantly elevated in glomeruli from both integrin α1-null mice and DKO mice compared to wild-type mice.
Figure 2.
Real-time PCR analysis of glomerular RNA from wild-type, Alport, DKO, and α1 integrin-deficient mice. Glomerular RNA from four independent sets of animals was analyzed for mRNA encoding the indicated MMPs. Data were normalized to GAPDH, which was run in multiplex with each sample. Differences in expression were significant if P < 0.05 (*, for comparison with wild-type mRNA levels; **, for comparison of Alport MMP-9 with α1−/− or DKO). WT, wild type; Alp, Alport; DKO, α1 integrin-deficient Alport (double knockout); α1−/−, α1 integrin-null.
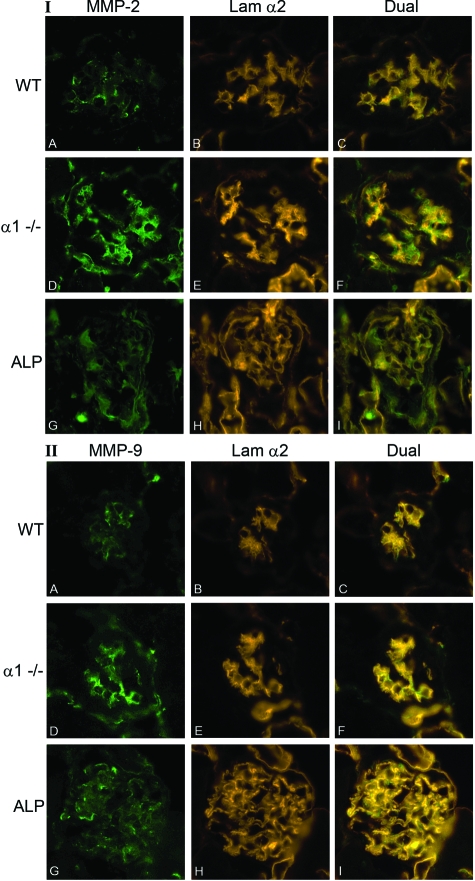
To address whether protein expression is also elevated in glomeruli from α1-null mice and Alport compared to wild-type mice, we performed immunofluorescence analysis using antibodies specific for MMP-2 and MMP-9 (we were unable to identify a suitable antibody for MMP-14). Age (7 weeks)- and strain-matched kidneys from wild-type, integrin α1-null, and Alport mice were embedded in the same blocks, allowing uniform immunostaining and processing. Immunostaining for the α2 chain of laminin was used as a marker for the glomerular mesangium for wild-type and integrin α1-null glomeruli. As mentioned, both the GBM and the mesangium are immunopositive for laminin α2 chain in Alport glomeruli. The results in Figure 3 show that immunostaining for both MMP-2 and MMP-9 localizes to the glomerular mesangium of both wild-type mice and integrin α1-null mice based on co-localization with laminin α2. Immunostaining is weak in wild-type glomeruli (Figure 3, IA and IIA), and robust in glomeruli from integrin α1-null mice (Figure 3, ID and IID). Cryosections from age-matched Alport mice were also examined, and show more widespread and diffuse immunolocalization, consistent with expanding mesangium in these mice. It is difficult to rule out podocyte contribution to elevated MMP-9 expression in Alport glomeruli. Immunostaining for MMP-9 is more robust in Alport glomeruli relative to wild type, consistent with real-time PCR results of RNA from isolated glomeruli (Figure 2). These results confirm higher expression of these MMPs in the glomeruli of α1-null animals compared to wild type, and suggest that mesangial cells of the glomerulus are the source of elevated MMP expression, at least for wild-type and α1-null mice.
Figure 3.
MMP-2 and MMP-9 immunostaining in glomeruli from wild-type, integrin α1-null, and Alport mice. Cryosections of wild-type mouse kidneys (A–C), α1 integrin-null mouse kidneys (D–F), and Alport kidneys (G–I) from 7-week-old mice were analyzed by immunofluorescence microscopy using antibodies specific for either MMP-2 (I: A, D, G) or MMP-9 (II: A, D, G). Laminin α2 was used as a marker for the mesangial matrix for WT and α1−/− immunostains (I and II: B and E); for Alp mice, laminin α2 localizes to both the mesangial matrix and the GBM. Dual immunostaining is shown in C, F, and I for both panels. WT, wild type; α1−/−, α1 integrin-null; Alp, Alport. Original magnifications, ×400.
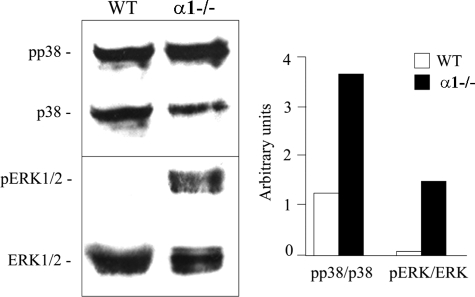
Absence of α1 integrin may influence collagen-mediated cell signaling events resulting in the up-regulation of gelatinase expression. Previous studies have linked the MAPK signaling pathway to collagenase expression and matrix remodeling.27 We examined MAPK activation status in isolated glomeruli of wild-type mice compared to integrin α1-null mice. Glomeruli were isolated from the 7-week-old mice, lysed in RIPA buffer, and analyzed by Western blot for activation of both the p38 and ERK 1/2 branches of the MAPK pathway. The results in Figure 4 show that ratios of pp38:p38 and pERK1/2:ERK 1/2 were elevated in glomerular extracts from integrin α1-null mice relative to wild-type controls.
Figure 4.
Both pERK and pp38 levels are elevated in isolated glomeruli from integrin α1-null mice relative to wild-type mice. Extracts of isolated glomeruli from wild-type and integrin α1-null mice were immunoblotted and probed with antibodies against the indicated components of the MAPK cascade. The ratios of phosphorylated to unphosphorylated proteins were measured by densitometry and are presented in bar graph format on the right. WT, Wild type; α1−/−, α1 integrin-null.
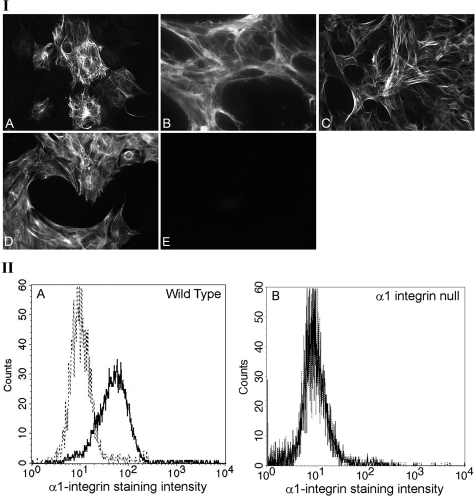
Isolated glomeruli are comprised of podocytes, endothelial cells, and mesangial cells in a matrix and cytokine-rich microenvironment. Thus, biochemical analysis of MAPK signaling in whole glomeruli will not likely reflect what is happening in the mesangial cells, even though they are the principal integrin α1-positive cell in the glomerulus (Figure 1). Because Figure 3 suggests mesangial cells are the principal source of elevated MMPs in integrin α1-null glomeruli, we derived primary mesangial cell cultures from wild-type, Alport, DKO, and integrin α1-null mice for further analysis. The cells were cultured from isolated glomeruli as described in the Materials and Methods. Figure 5 shows qualification results from a typical preparation. Figure 5I shows that the cells were immunopositive for desmin, fibronectin, smooth muscle actin, and vimentin (Figure 5, A–D, respectively), accepted markers for mesangial cells.22 They were negative for von Willebrand factor, a marker for endothelial cells (Figure 5E). They were also immunonegative for podocin, nephrin, and CD2AP, suggesting an absence of podocyte contamination (data not shown). Because these cells are cultured, it is important to confirm that the wild-type cells express α1β1 integrin. Cells were stained with a monoclonal antibody specific for α1β1 integrin,5 or an isotype-matched nonreactive antibody and analyzed by fluorescence-activated cell sorting. Figure 5II shows that the wild-type cells are immunopositive for α1β1 integrin (Figure 5IIA), whereas the α1 integrin-null cells express no α1β1 integrin (Figure 5IIB). These results confirm that the mesangial cell culture system we have established should allow us to explore the role of α1β1 integrin in regulating gelatinase expression.
Figure 5.
Qualification of primary mesangial cell cultures. I: Immunostaining results for a typical primary mesangial cell preparation is shown. A, Anti-desmin; B, anti-fibronectin; C, anti-smooth muscle actin; D, anti-vimentin; E, anti-von Willebrand factor (a marker for endothelial cells). Cells were negative for podocyte markers podocin, nephrin, and CD2AP (not shown). II: FACS analysis of wild-type and integrin α1-null mesangial cells using anti-α1 integrin antibodies. Primary cultured mesangial cells from either wild-type (IIA) or integrin α1-null (IIB) mice were reacted with either a monoclonal antibody specific for α1 integrin (solid lines) or a nonreactive isotype-matched control antibody (dashed lines). After reaction with a fluorescein isothiocyanate-conjugated secondary antibody, immunopositive cells were detected by fluorescence-activated cell sorting. Histograms represent a gated population of live mesangial cells and are representative of three independently conducted experiments from distinct culture derivations. Original magnifications, ×630.
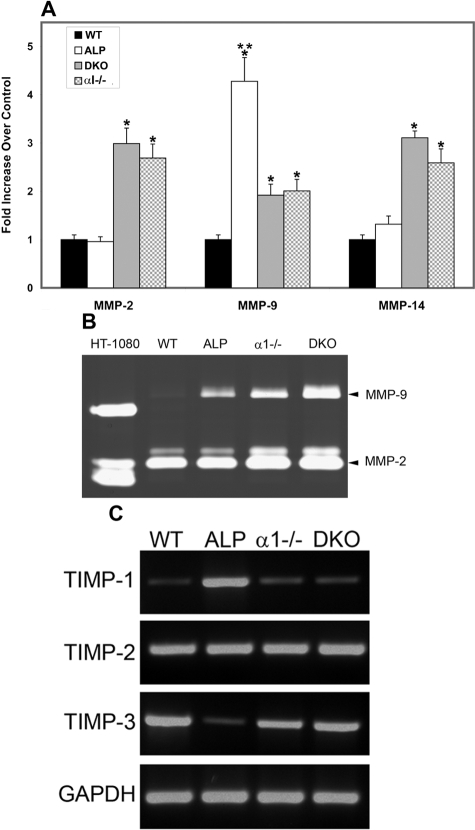
RNA from wild-type, Alport, DKO, and integrin α1-null mesangial cells cultured on type I collagen was analyzed for expression of MMP-2, MMP-9, and MMP-14 using real-time RT-PCR. Four independent preparations of mesangial cells were analyzed in triplicate. The results in Figure 6A illustrate that mRNAs encoding all three MMPs are significantly elevated in mesangial cells from integrin α1-null mice and DKO mice compared to wild-type controls. Cultured mesangial cells from Alport mice showed significantly elevated levels of MMP-9 compared to wild-type cells, but normal expression levels of MMP-2 and MMP-14. These data are nearly identical to results obtained with isolated glomeruli (Figure 2), suggesting that dysregulation of gelatinase expression in α1 integrin-null and Alport glomeruli emanates from the mesangial cell compartment. Gelatinase activity in serum-free media conditioned by these same cultures was assessed using gelatin zymography. Figure 6B shows gelatinase activity for both MMP-2 (both the pro and active isoforms) and MMP-9 was elevated in medium conditioned by α1-null mesangial cells compared to wild-type mesangial cells. Cultured mesangial cells from Alport mice showed elevated MMP-9, but not MMP-2. Interestingly, although MMP-9 mRNA in Alport mesangial cells was higher than that in α1-null mesangial cells, MMP-9 activity was considerably lower in media from Alport versus integrin α1-null cells. This may indicate translational or secretory differences in cultured mesangial cells from the two mouse models. Medium conditioned by HT-1080 cells (a human fibrosarcoma cell line) was used as a positive control (human MMPs run slightly faster than mouse MMPs). The zymography presented was qualitatively consistent with similar zymographs from three other different independently derived sets of cells.
Figure 6.
Analysis of MMP mRNA expression, MMP activity, and TIMP expression in primary mesangial cells from wild-type, Alport, α1-null, and DKO mice. A: Mesangial cell RNA prepared from four independent sets of 6-week-old animals were analyzed in triplicate for mRNA encoding MMP-2, MMP-9, and MMP-14. Data were normalized to GAPDH, which was run in multiplex with each sample. Asterisks denote statistically significant differences (P < 0.005). *MMP induction relative to wild-type cells; **MMP-9 induction in Alport cells relative to α1-null and DKO cells. B: Serum-free media conditioned by wild-type (WT), Alport (ALP), integrin α1-null (α1−/−), or DKO mesangial cells were analyzed for MMP activity by gelatin zymography. Media concentration was normalized to total cell protein before loading. Conditioned media from HT1080 cells was used as a positive control (human MMP-9 runs slightly faster than mouse MMP-9). The gel shown is highly representative of results observed for three sets of independent mesangial cell derivations. C: TIMP mRNA expression from mesangial cell cultures was analyzed using RT-PCR. The bottom row of bands shows mRNA for glyceraldeyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, which was used as a loading control. WT, wild type; ALP, Alport; α1−/−, α1 integrin-null; DKO, integrin α1-null Alport.
The endogenous inhibitors of the MMPs influence MMP activity in vivo. We examined mRNA expression of TIMP-1, -2, -3, and -4 in wild-type (WT), Alport (ALP), integrin α1-null (α1−/−), and integrin α1-null Alport (DKO) mesangial cells using RT-PCR. The results in Figure 6C show that TIMP-1 is markedly elevated in Alport mesangial cells relative to wild-type cells, and TIMP-3 mRNA is markedly decreased in Alport mesangial cells relative to wild-type cells. These results are surprisingly similar to those we reported earlier for total kidney RNA using Northern blots.25 In integrin α1-null Alport (DKO) cells, the effect of the Alport mutation was ameliorated. Like α1-null cells, DKO cells did not differ significantly from wild-type cells for expression of TIMPs. There was no effect of either Alport or α1-null genotype on TIMP-2 gene expression in cultured mesangial cells. TIMP-4 was not expressed in any of the mesangial cell cultures (data not shown).
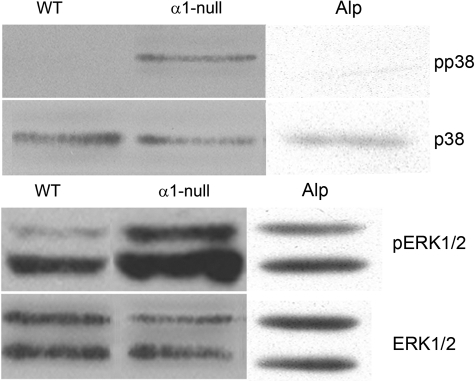
The activation status of the MAPK signal transduction pathway was analyzed in cultured mesangial cells from wild-type, integrin α1-null, and Alport mice by Western blot analysis using antibodies specific for pp38/p38 or pERK/total ERK. The results in Figure 7 illustrate that both the p38 and ERK branches of the MAPK signal transduction pathway are activated (based on ratios of pp38/p38 and pERK/ERK) in mesangial cells from integrin α1-null mice relative to wild-type mice, data that is again consistent with that observed in isolated glomeruli (Figure 4). Alport mesangial cells show no difference in either p38 or ERK activation compared to wild-type cells.
Figure 7.
Mesangial cells from α1-null mice, but not Alport mice, have elevated levels of pp38 and pERK relative to wild-type mice. Cell lysates from wild-type (WT), integrin α1-null (α1-null), and Alport (Alp) mesangial cell cultures were electrophoresed and transferred to PVDF membranes and phosphorylated ERK 1/2 (pERK 1/2), total ERK 1/2 (ERK 1/2), phosphorylated p38 (pp38), or total P38 (p38) was detected by Western blotting using specific antibodies. Qualitatively similar results were observed using lysates from three independently derived sets of cultures.
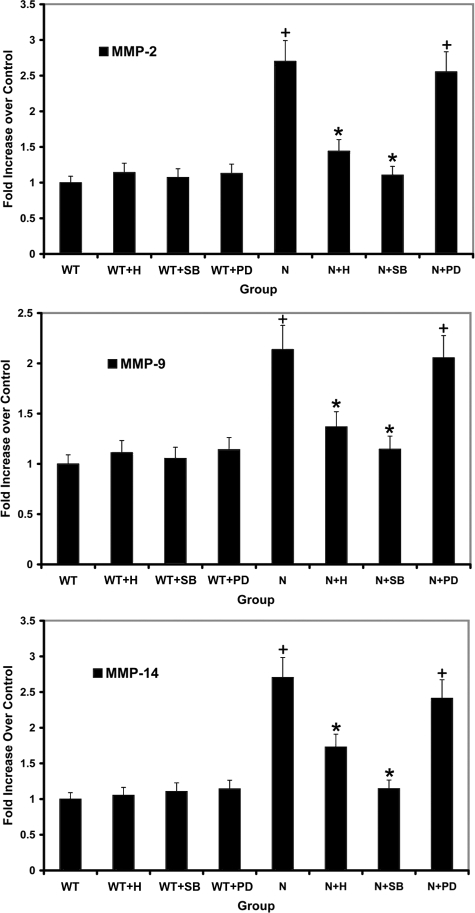
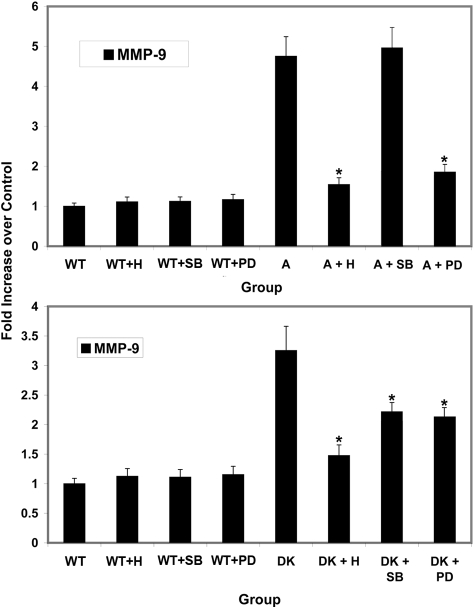
Previous studies have linked both the ERK and p38 branches of MAPK with regulation of MMPs.14,28 We used a series of small molecule inhibitors aiming to determine whether one or both branches of MAPK are regulating MMP-2, -9, and -14. Mesangial cells were either untreated or treated with herbimycin (inhibits both branches of MAPK), PD 98059 (inhibits the ERK1/2 branch of MAPK), or SB 203580 (inhibits p38 branch of MAPK). RNA was isolated and analyzed for expression levels of MMP-2, MMP-9, and MMP-14 using real-time RT-PCR. RNA from four independently isolated primary mesangial cell cultures were analyzed in triplicate. The results in Figure 8 show that blocking the p38 branch of MAPK in α1 integrin-null cells brings mRNA expression levels of MMP-2, MMP-9, and MMP-14 down to levels observed in wild-type controls. In contrast, blocking the ERK branch of MAPK had no effect on expression of these same MMPs. Blocking both branches with herbimycin had an intermediate effect, likely attributable to partial inhibition. An identical experimental design strategy was used using cells from Alport mice and DKO mice to determine whether the MAPK pathway regulates MMP-9 in these cells. In mesangial cells from Alport mice, elevated MMP-9 expression was unaffected by the p38 MAPK inhibitor, whereas induction of MMP-9 was completely abolished with the ERK MAPK inhibitor (Figure 9, top). For integrin α1-null Alport (DKO) mesangial cells, both p38 MAPK and ERK MAPK signaling influenced MMP-9 expression (Figure 9, bottom).
Figure 8.
p38 MAP kinase but not ERK 1/2 kinase is required for increased expression of MMP-2, MMP-9, and MMP-14 in mesangial cells from α1-integrin-deficient mice. Mesangial cells were incubated for 24 hours in the presence of herbimycin (1.0 μmol/L), SB 203580 (p38 inhibitor, 10 μmol/L), or PD 98059 (ERK 1/2 inhibitor, 40 μmol/L). RNA prepared from three independently derived sets of mesangial cells was analyzed in triplicate for mRNA encoding MMP-2, MMP-9, and MMP-14 using real-time PCR. Data were normalized to GAPDH, which was run in multiplex with each sample. Asterisks denote statistically significant differences (P > 0.05) in specific MMP expression when comparing: +normal and integrin α1-null mesangial cell RNA; *drug-treated α1-null and vehicle-treated α1-null mesangial cell RNA. C, control; N−/−, α1 integrin-null; H, herbimycin; PD, PD 98059; and SB, SB 203580.
Figure 9.
Increased expression of MMP-9 in Alport mesangial cells is regulated by ERK1/2 MAP kinase but not p38 MAP kinase. The same experimental strategy used in Figure 8 was used to characterize MMP-9 regulation in mesangial cells from Alport mice (top) and integrin α1-null Alport mice (bottom). RNA prepared from three independently derived sets of mesangial cells was analyzed in triplicate. Data were normalized to GAPDH, which was run in multiplex with each sample. Asterisks denote statistically significant differences in specific MMP expression when comparing results from vehicle-treated Alport or DKO mesangial cells with drug-treated Alport or DKO mesangial cells (P > 0.05). Wt, wild type; A, Alport; DK, α1-null Alport; H, herbimycin; PD, PD 98059; SB, SB 203580.
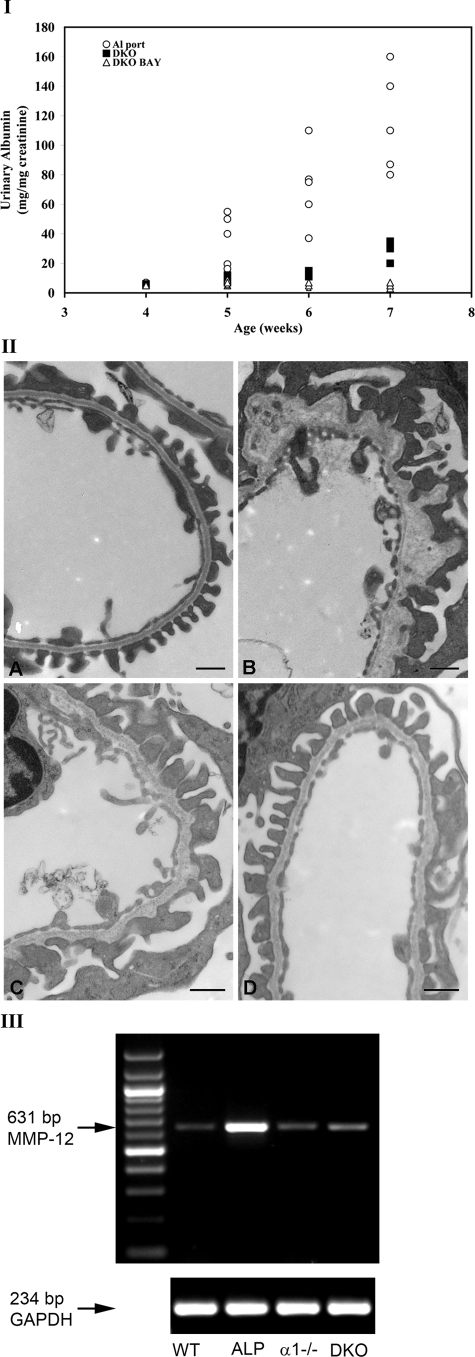
We surmised that attenuated glomerular disease in Alport mice on an integrin α1-null background might be linked to elevated gelatinase expression (MMP-2, MMP-9, and MMP-14) resulting from the absence of integrin α1β1. To test this hypothesis we used the matrix metalloproteinase inhibitor BAY-12-9566. This compound has selective substrate specificity for MMPs, with Ki in the nM range for MMP-2 and MMP-9.29,30 BAY-12-9566, or a carboxymethylcellulose vehicle was administered by oral gavage to wild-type mice or integrin α1-null Alport mice starting at 4 weeks of age until 7 weeks of age. Three sets of animals were analyzed in triplicate. We chose to measure albuminuria as a measure of GBM integrity. Urine was collected at weekly intervals and analyzed for albuminuria normalized to urinary creatinine using QuantiChrome colorimetric assays (BioAssay Systems). Figure 10I shows that the MMP inhibitor significantly reduced the progression of albuminuria across all time points in DKO mice given BAY-12-9566 compared to DKO mice given only carboxymethylcellulose carrier. Similar measures for Alport mice are included as reference, demonstrating reduced proteinuria in DKO mice relative to Alport mice as previously documented.3 We examined the effect of BAY-12-9566 treatment on GBM ultrastructure in these same mice using transmission electron microscopy. Figure 10II shows that GBM architecture in treated mice (Figure 10IID) is improved compared to carboxymethylcellose-treated control DKO mice (Figure 10IIC) resulting in GBM architecture similar to wild-type mice (Figure 10IIA). Age-matched (7 weeks) Alport GBM architecture is provided for a reference (Figure 10IIB). We previously reported that BAY-12-9566 treatment had no measurable effect on Alport renal disease progression.24These data suggest that elevated glomerular MMP expression imparted by the α1-null genotype actually exacerbated glomerular disease progression in DKO mice, suggesting that the α1-null genetic background has both positive3 and negative (shown here) effects on the progression of glomerular disease in these mice.
Figure 10.
Treatment of integrin α1-null Alport (DKO) mice with BAY-12-9566 ameliorates the progression of albuminuria, restoring relatively normal GBM architecture. I: Wild-type mice and DKO mice were treated daily with BAY-12-9566 or carboxymethylcellulose carrier starting at 4 weeks of age until 7 weeks of age. Untreated Alport mice were included as a reference for reduced proteinuria in DKO mice. Three to five sets of animals were analyzed in triplicate. Urine was collected at weekly intervals and analyzed for urinary albumin and normalized to urinary creatinine using commercial quantitative colorimetric plate assays (see Materials and Methods). DKO, integrin α1-null Alport; DKO BAY, DKO treated with BAY-12-9566. II: Transmission electron microscopic analysis of GBM architecture from 7-week-old wild-type (A), Alport (B), vehicle-treated (from 4 to 7 weeks) DKO (C), and BAY-12-9566-treated (4 to 7 weeks) DKO (D) mice. III: MMP-12 induction is ameliorated in DKO mice relative to Alport mice. Glomerular RNA from 7-week-old wild-type (WT), Alport (ALP), integrin α1-null (α1−/−), and integrin α1-null Alport (DKO) mice was analyzed for MMP-12 mRNA using RT-PCR. The bottom row of bands shows mRNA for glyceraldeyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, which was used as a loading control. Scale bars = 500 nm.
These observations leave unanswered the question regarding why the integrin α1-null genotype results in markedly slowed progression of Alport glomerular disease as noted previously.3 In a recent report we demonstrated a mechanistic link between metalloelastase (MMP-12) expression and GBM damage in Alport mice.24 Given the obvious effects of α1 integrin-null background on MMP-2, -9, and -14 demonstrated in this work, we surmised that MMP-12 expression might also be influenced by the α1 integrin-null background in Alport mice. To address this, we examined MMP-12 expression in glomerular RNA from 7-week-old wild-type, Alport, integrin α1-null, and DKO mice using RT-PCR. The results in Figure 10III show that MMP-12 expression in Alport glomeruli is markedly induced relative to wild-type glomeruli as previously reported.24 The α1-null background results in amelioration of MMP-12 induction in DKO mice to levels comparable to wild-type mice. This observation may account, in part, for the observed improvement of glomerular function in α1 integrin-null Alport mice. Reminiscent to the results shown in Figure 6C, these findings collectively suggest a complex effect of the α1-null background on the proteolytic machinery of the renal glomerulus in Alport mice, with elevated expression of some MMPs (MMP-2, -9, and -14) and suppression of potentially deleterious dysregulation of other molecular players in matrix homeostasis (MMP-12, TIMP-1, and TIMP-3).
Discussion
In an earlier study we showed that neutralization of integrin α1β1 reduces glomerular pathology and nearly doubles the life span in the Alport mouse model.3 The specific mechanism(s) underlying this observation have remained obscure. The present study was undertaken in an attempt to shed light on how α1β1 integrin influences extracellular matrix homeostasis in glomeruli. We show that the expression of MMP-2, MMP-9, and MMP-14 are significantly elevated in both isolated glomeruli and in cultured primary mesangial cells from integrin α1β1-deficient mice compared to wild-type mice. Elevated MMP-2, MMP-9, and MMP-14 expression is linked to activation of the p38 branch of the MAPK signaling pathway. As inferred from the specificity of the inhibitor used in these studies (SB 203580), regulation of these MMPs involves either the α or β isoforms of p38.31 In contrast, both glomeruli and mesangial cells from Alport mice showed elevated levels of MMP-9, but not MMP-2 or MMP-14. In this case, treatment of Alport mesangial cell cultures with MAPK inhibitors revealed MMP-9 regulation by the ERK signaling pathway. In mesangial cells from integrin α1-null Alport mice, both ERK and P38 MAPK show regulatory influence on MMP-9 expression. Treatment of α1-null Alport mice with MMP-inhibitor BAY-12-9566 from 4 to 7 weeks of age ameliorated the progressive increase in proteinuria, inferring improved glomerular function. Thus, in addition to its beneficial effects the α1 integrin-null background causes imbalances in MMP expression that are deleterious to glomerular health. This may help explain why the α1 integrin-null background results in exacerbated glomerular injury in diabetic mice,32 and after glomerular injury with adriamycin.6
On the other hand we also show that the α1-null background has a neutralizing effect on other important regulators of basement membrane homeostasis, MMP-12, TIMP-1, and TIMP-3. Suppression of MMP-12 expression in the α1-null background would certainly contribute to attenuated Alport glomerular disease progression based on recently published work showing that MMP-12 contributes to GBM destruction in Alport syndrome.24 TIMP-1 is widely thought to be the main endogenous inhibitor of MMP-9 based on its binding affinity for MMP-9,33,34 so the observed marked induction of TIMP-1 would likely reduce the effects of MMP-9 induction in Alport glomeruli. In DKO glomeruli, suppression of TIMP-1 induction might exacerbate the effects of elevated MMP-9 expression. TIMP-3 inhibits MMP-1, -2, -3, and -14.35 We show suppression of TIMP-3 in Alport mesangial cells, which would likely result in elevated proteolytic activity from its target MMPs. In DKO mesangial cells, suppression of TIMP-3 expression is not observed, which would be expected to restore the normal balance of TIMP-3 activity with its MMP targets. Overall, our findings suggest the α1-null background results in a complex effect on the proteolytic machinery affecting matrix metabolism in the Alport renal glomerulus. This multigenic effect would be expected to influence different glomerular disease models in distinct ways.
The role of MMPs in Alport glomerular pathogenesis has been explored previously. MMP-9 (gelatinase B) knockout mice were crossed with Alport mice, and the double mutants showed no influence of MMP-9 on glomerular disease progression.36 Studies using a small molecule inhibitor cocktail specific for MMP-2, MMP-3, and MMP-9 showed that Alport glomerular function was improved if the drugs were administered early in the disease progression, and exacerbated if the drugs were administered late in the disease progression.37 These experiments were performed using Alport mice on the C57BL/6 background, and are in contrast to similar experiments performed on the 129 Sv/J background, which show no effect of BAY-12-9566 on renal disease progression in Alport mice.24 These two mouse strains differ in GBM composition because of a strain-dependent type IV collagen isoform switch, which results in the expression of an atypical collagen network in the GBM comprised of α5(IV)/α6(IV) chains.38 A recent review addressed strain consideration in Alport mice.39 It is likely that this strain-related difference in type IV collagen composition of the GBM contributes to the prolonged lifespan of Alport mice on the C57BL/6 background compared to Alport mice on the 129 Sv/J background; however, a quantitative trait locus has also been correlated with prolonged lifespan in C57BL/6 Alport mice.40 There is little doubt that the atypical collagen network could influence glomerular gene expression. For example, MMP-2 is elevated in glomeruli of C57BL/6 Alport mice37 but not 129 Sv/J Alport mice (this study),24 quite possibly owing to the different collagen composition of the GBM in the two strains. Indeed this difference might explain why gelatinase inhibitors ameliorate disease progression in C57BL/6 Alport mice but not 129 Sv/J mice.24,37 In this study we show that a gelatinase inhibitor improves glomerular function in integrin α1-null Alport mice, which are on the 129 Sv/J background. These mice are similar to C57BL/6 Alport mice with respect to elevated expression of MMP-2. Collectively, these data suggest MMP-2 might be an important modulator of GBM disease progression in Alport mouse models in which elevated expression of MMP-2 is observed. Definitive work linking MMP-2 to Alport glomerular disease is still lacking.
Both the ERK and P38 branches of the MAPK pathway have been implicated in MMP regulation in both cultured mesangial cells and in glomerular disease states.14,28 The signaling cascades involved appear to function via cross talk with growth factor signaling mechanisms including transforming growth factor-β, epidermal growth factor, and platelet-derived growth factor.41,42,43 Normal mesangial matrix remodeling requires a delicate balance of synthesis and degradation, and an imbalance in this process can lead to mesangial matrix accumulation, impeding the normal function of the mesangial cells and contributing to the progression of certain glomerular diseases.
Deletion of the α1 integrin subunit by targeted mutagenesis results in a mouse with no obvious discernable phenotype.10 Through the use of this model the collagen binding integrin α1β1 has since been shown to be an important mediator of angiogenesis and tumor growth.11,44 Earlier work from this and other laboratories have also clearly implicated a role for α1β1 integrin in chronic inflammatory diseases using both integrin α1-null mice and neutralizing antibodies against α1β1 integrin. The work was initiated in studies using the Alport mouse model3 and expanded to include models for chronic inflammatory bowel disease,45 rheumatoid arthritis,46 crescentic nephritis,47 and anti-Thy1 nephritis.41 Most of the leukocytes recruited in these inflammatory diseases were immunopositive for α1β1 integrin,48 even though α1β1-positive leukocytes constitute only a minor fraction of the peripheral blood leukocyte population. Whether this observation is attributable to selective recruitment, selective proliferation, or some combinations of these is not yet known. Nonetheless, when these studies are viewed in the context of the findings presented here, it appears that the effects of a α1-null background on Alport renal pathogenesis is multifaceted, affecting (at least) matrix remodeling in the glomerulus, and monocyte recruitment in the tubulointerstitium. Clearly the α1-null background results in constitutively elevated metalloproteinase expression in the mesangium, and altered MAPK signaling suggests that it likely affects expression of other genes as well. This broader influence of the integrin α1-null background on glomerular function might contribute toward explaining why integrin α1-null mice have an increased susceptibility to adriamycin-induced glomerulosclerosis than wild-type mice.6 This observation, when viewed in the context of α1-null influence on Alport renal disease progression, suggests absence of α1 integrin affects the progression of specific renal diseases differently, and can result in either acceleration or attenuation of disease progression. Further analysis of the apparent pleotrophic effects of α1β1 integrin signaling in the normal and diseased glomerulus might provide clues regarding the important molecular contributors to glomerular disease progression in these different disease models.
Mesangial cell matrix/integrin interactions have been implicated in mesangial cell migration and proliferation, maintenance of the glomerular capillary ultrastructure, and preventing mesangial cell apoptosis.49,50 In addition to roles for α1β1 integrin in mesangial cell function, α5β1 has been implicated as a regulator of both transforming growth factor-β1 and plasminogen activator inhibitor-1, suggesting a role for integrin α1β1 in mesangial matrix remodeling.51 Besides the β1 integrins, integrin αVβ3/vitronectin interactions in the mesangium have been implicated as contributing to mesangial matrix expansion in diabetic nephropathy.52
There has been a significant amount of work done regarding the role of α1β1 integrin in mesangial matrix remodeling. Much of this work has focused on the use of collagen gel contraction assays as a means of demonstrating effects on mesangial matrix remodeling mechanisms. Mesangial cells cultured in the type I collagen gel fail to contract the gel when cultured in the presence of neutralizing antibodies against either integrin α1 or β1 subunits.53 Administration of these same neutralizing antibodies to anti-thy-1 nephritic rats reduced mesangial hypercellularity and mesangial matrix accumulation, suggesting that the hypothesis in which α1β1 signaling influences mesangial matrix remodeling in disease states has in vivo relevance. Transforming growth factor-β1 up-regulates α1β1 integrin on mesangial cells, increasing adhesion to collagen, migratory potential, and gel contraction.27,53 Platelet-derived growth factor treatment enhanced migration and gel contraction without influencing α1β1 integrin expression in this system, via activation of the ERK branch of MAP kinase.9,27 This further exemplifies the pleotrophic effects of α1β1 integrin signaling on mesangial cell behavior in healthy and diseased glomeruli.
The constitutive activation of MAP kinase signaling in the absence of α1β1 integrin likely results from decreased cell adhesion, suggesting cross talk between focal adhesion kinase and/or integrin-linked kinase systems and the MAP kinase signaling pathway. Such cross talk has been documented, but never in mesangial cells, and never in relation to regulation of MMP expression.54 Future studies will be aimed at further defining the complex nature of α1β1 integrin-mediated regulation of MMP expression.
In summary, α1 integrin-null Alport mice show markedly attenuated glomerular disease progression.3 In this work we show that the α1 integrin-null background results in significantly elevated expression of gelatinases in both glomeruli and cultured mesangial cells. Inhibiting these gelatinases in α1-integrin-null Alport mice slowed the progression of albuminuria, suggesting that elevated gelatinases promote glomerular disease in this model. On the other hand, the α1-null background results in ameliorated induction of MMP-12 and TIMP-1, as well as ameliorated suppression of TIMP-3. MMP-12 induction has been linked to GBM destruction associated with Alport syndrome,24 and TIMP-3 suppression might result in elevated activity of its MMP targets,35 resulting in further GBM destruction. Therefore, the lack of α1 integrin in Alport mice results in molecular changes that both promote (elevated gelatinase activity), and protect (ameliorated induction ofMMP-12, and possibly ameliorated suppression of TIMP-3) against glomerular disease progression. These complex pleotrophic effects of α1 integrin on the proteolytic machinery that regulates matrix metabolism would suggest that neutralization of α1 integrin would affect different glomerular disease models in distinct ways. This is indeed what has been observed thus far.3,6,32
Acknowledgments
We thank John (Skip) Kennedy for help in figure preparation.
Footnotes
Address reprint requests to Dominic Cosgrove, Ph.D., Director of Basic Research, Boys Town National Research Hospital, 555 No. 30th St., Omaha, NE 68131. E-mail: cosgrove@boystown.org.
Supported by the National Institutes of Health (grants R01 DK55000 and R01 DC04844 to D.C. and RO1 CA94849-01 to A.P.).
References
- Kashtan CE. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine. 1999;78:338–360. doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Kashtan CE. Animal models of Alport syndrome. Nephrol Dial Transplant. 2002;17:1359–1361. doi: 10.1093/ndt/17.8.1359. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M, Ylänne J, Laitinen L, Virtanen I. The α1-α6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ. Glomerular epithelial and mesangial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab Invest. 1995;72:367–375. [PubMed] [Google Scholar]
- Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol. 2004;165:617–630. doi: 10.1016/s0002-9440(10)63326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Kondo S, Loster K, Reutter W, Kuhara T, Yasutomo K, Kuroda Y. Alpha1beta1 integrin-mediated collagen matrix remodeling by rat mesangial cells is differentially regulated by transforming growth factor-beta and platelet-derived growth factor-BB. J Am Soc Nephrol. 1999;10:779–789. doi: 10.1681/ASN.V104779. [DOI] [PubMed] [Google Scholar]
- Kagami S, Kondo S, Urushihara M, Loster K, Reutter W, Saijo T, Kitamura A, Kobayashi S, Kuroda Y. Overexpression of alpha1beta1 integrin directly affects rat mesangial cell behavior. Kidney Int. 2000;58:1088–1097. doi: 10.1046/j.1523-1755.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- Kagami S, Urushihara M, Kondo S, Loster K, Reutter W, Tamaki T, Yoshizumi M, Kuroda Y. Requirement for tyrosine kinase-ERK1/2 signaling in alpha 1 beta 1 integrin-mediated collagen matrix remodeling by rat mesangial cells. Exp Cell Res. 2001;268:274–283. doi: 10.1006/excr.2001.5279. [DOI] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloproteinase and angiostatin levels in integrin a1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21:272–281. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- Urushihara M, Kagami S, Kuhara T, Tamaki T, Kuroda Y. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol Dial Transplant. 2002;17:1189–1196. doi: 10.1093/ndt/17.7.1189. [DOI] [PubMed] [Google Scholar]
- Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000;165:5788–5797. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Boustead JN, Chapman SC, Svitek CA, Oeser JK, Robey RB, O'Brien RM. Insulin-mediated activation of activator protein-1 through the mitogen-activated protein kinase pathway stimulates collagenase-1 gene transcription in the MES 13 mesangial cell line. J Mol Endocrinol. 2004;33:263–280. doi: 10.1677/jme.0.0330263. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Kotelianski V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JI, Hoover RL, Karnovsky MJ. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978;14:21–30. doi: 10.1038/ki.1978.86. [DOI] [PubMed] [Google Scholar]
- Harper PA, Robinson JM, Hoover RL, Wright TC, Karnovsky MJ. Improved method for culturing rat glomerular cells. Kidney Int. 1984;26:875–880. doi: 10.1038/ki.1984.231. [DOI] [PubMed] [Google Scholar]
- Gilbert SF, Migeon BR. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 1975;5:11–17. doi: 10.1016/0092-8674(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Menè P. Mesangial cell cultures. J Nephrol. 2000;14:198–203. [PubMed] [Google Scholar]
- Gratton MA, Rao VH, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of Alport mice: implications for capillary basement membrane pathology. Am J Pathol. 2005;166:1465–1474. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VH, Meehan D, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D. Role for MMP-12 in GBM damage associated with Alport syndrome. Am J Pathol. 2006;169:32–46. doi: 10.2353/ajpath.2006.050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D. Increased expression of MMP-2. MMP-9 (type IV collagenases/gelatinases) and MT1-MMP in canine X-linked Alport syndrome (XLAS). Kidney Int. 2003;63:1736–1748. doi: 10.1046/j.1523-1755.2003.00939.x. [DOI] [PubMed] [Google Scholar]
- Rodgers KD, Rao V, Meehan DT, Fager N, Gotwals P, Ryan ST, Koteliansky V, Nemori R, Cosgrove D. Tissue monocytes may promote myofibroblast accumulation and tubular epithelial cell death in renal fibrosis. Kidney Int. 2003;63:1338–1355. doi: 10.1046/j.1523-1755.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- Kagami S, Urushihara M, Kitamura A, Kondo S, Hisayama T, Kitamura M, Loster K, Reutter W, Kuroda Y. PDGR-BB enhances alpha1beta1 integrin-mediated activation of the ERK/AP-1 pathway involved in collagen matrix remodeling by rat mesangial cells. J Cell Physiol. 2004;198:470–478. doi: 10.1002/jcp.10433. [DOI] [PubMed] [Google Scholar]
- Tack I, Elliot SJ, Potier M, Rivera A, Striker GE, Striker LJ. Autocrine activation of the IGF-I signaling pathway in mesangial cells isolated from diabetic NOD mice. Diabetes. 2002;51:182–188. doi: 10.2337/diabetes.51.1.182. [DOI] [PubMed] [Google Scholar]
- Gatto C, Rieppi M, Borsotti P, Innocenti S, Ceruti R, Drudis T, Scanziani E, Casazza AM, Taraboletti G, Giavazzi R. BAY 12-9566. A novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin Cancer Res. 1999;5:3603–3607. [PubMed] [Google Scholar]
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:179–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Dev. 2005;8:421–430. [PubMed] [Google Scholar]
- Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A. Gomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- Murphy G, Willenbrock F, Crabbe T, O'Shea M, Ward R, Atkinson S, O'Conell J, Docherty A. Regulation of matrix metalloproteinase activity. Proc Natl Acad Sci USA. 732:31–41. doi: 10.1111/j.1749-6632.1994.tb24722.x. [DOI] [PubMed] [Google Scholar]
- Roeb E, Behrmann I, Grötzinger , Breuer B. An MMP-9 mutant without gelatinolytic activity as a novel TIMP-1-antagonist. FASEB J. 2000;14:1671–1673. doi: 10.1096/fj.99-0947fje. [DOI] [PubMed] [Google Scholar]
- Wei S, Kashiwagi M, Kota S, Xie Z, Nagase H, Brew K. Reactive site mutations in tissue inhibitor of metalloproteinase-3 disrupt inhibition of matrix metalloproteinases but not tumor necrosis factor-α-converting enzyme. J Biol Chem. 2005;280:32877–32882. doi: 10.1074/jbc.C500220200. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH. Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. Am J Pathol. 2000;157:303–311. doi: 10.1016/S0002-9440(10)64541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Khurana M, Sugimoto H, Cosgrove D, Rao VH, Rougier J-P, Werner MC, Shield CF, III, Werb Z, Kalluri R. Stage specific action of matrix metalloproteinases influence hereditary kidney disease. PLoS Med. 2006;3:535–546. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Baorza DB. Loss of alpha3/alpha4(IV) collagen from glomerular basement membrane induces a strain-dependent isoform switch to alpha5/alpha6(IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol. 2006;17:1962–1969. doi: 10.1681/ASN.2006020165. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Kalluri R, Miner J, Segal Y, Borza D-B. Choosing a mouse model to study the molecular pathobiology of Alport glomerulonephritis. Kidney Int. 2007;71:615–618. doi: 10.1038/sj.ki.5002115. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Mudd JL, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Urushihara M, Kondo S, Hayashi T, Yamano H, Loster K, Vossmeyer D, Reutter W, Kuroda Y. Effects of anti-alpha1 integrin subunit antibody on anti-Thy-1 glomerulonephritis. Lab Invest. 2002;82:1219–1227. doi: 10.1097/01.lab.0000027835.77351.bf. [DOI] [PubMed] [Google Scholar]
- Uchiyama-Tanaka Y, Matsubara H, Mori Y, Kosaki A, Kishimoto N, Amano K, Higashiyama S, Iwasaka T. Involvement of HB-EGF and EGF receptor transactivation in TGF-beta-mediated fibronectin expression in mesangial cells. Kidney Int. 2002;62:799–808. doi: 10.1046/j.1523-1755.2002.00537.x. [DOI] [PubMed] [Google Scholar]
- Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schager-Korting M, Pfeilschifter J, Huwiler A. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Nyberg P, Venkateshwar G, Keshamouni AP, Mannam JL, Sugimoto H, Cosgrove DE, Kalluri R. Extracellular matrix human alpha 1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated through alpha 1 beta 1 integrin. J Clin Invest. 2005;115:2801–2810. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Krieglstein CF, Cerwinka WH, Sprague AG. Collagen-binding integrin alpha 1 beta 1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:773–782. doi: 10.1172/JCI200215256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins α1β1 and α2β1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to α1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol. 2002;161:1265–1272. doi: 10.1016/S0002-9440(10)64403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson NS, Ryan ST, Enke DA, Cosgrove D, Koteliansky V, Gotwals P. Global gene expression analysis reveals a role for the α1 integrin in renal pathogenesis. J Biol Chem. 2001;276:34182–34188. doi: 10.1074/jbc.M102859200. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, Makino H. Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int. 1998;54:1188–1196. doi: 10.1046/j.1523-1755.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Pröls F, Hartner A, Schocklmann HO, Sterzel RB. Mesangial cells and their adhesive properties. Exp Nephrol. 1999;7:137–146. doi: 10.1159/000020594. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Ishimura E, Koyama H, Tanaka S, Imanishi Y, Shioi A, Inaba M, Nishizawa Y. Blocking of alpha 5 integrin stimulates production of TGF-beta and PAI-1 by human mesangial cells. Biochem Biophys Res Commun. 2003;305:815–819. doi: 10.1016/s0006-291x(03)00860-x. [DOI] [PubMed] [Google Scholar]
- Yoon S, Ginras D, Bendayan M. Alterations of vitronectin and its receptor alpha(v) integrin in the rat renal glomerular wall during diabetes. Am J Kidney Dis. 2001;38:1298–1306. doi: 10.1053/ajkd.2001.29228. [DOI] [PubMed] [Google Scholar]
- Kagami S, Kuhara T, Yasutomo K, Okada K, Löster K, Reutter W, Kuroda Y. Transforming growth factor-beta (TGF-beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Exp Cell Res. 1996;25:1–6. doi: 10.1006/excr.1996.0336. [DOI] [PubMed] [Google Scholar]
- Kapur R, Cooper R, Zhang L, Williams DA. Cross-talk between α4β1/α5β1 and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood. 2001;97:1975–1981. doi: 10.1182/blood.v97.7.1975. [DOI] [PubMed] [Google Scholar]