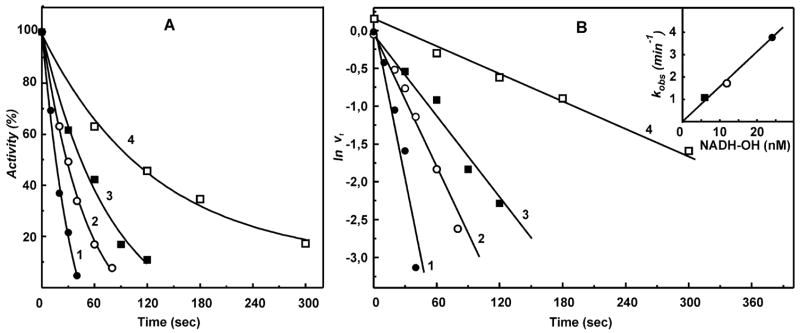
Figure 1.
Inhibition of the NADH oxidase activity by NADH-OH. (A) SMPs (5 μg of protein/mL, about 0.5 nM complex I) were incubated with 6 (■), 12 (○), and 24 (●, □) nM NADH-OH for the time indicated on the abscissa in the standard reaction mixture (0.25 M sucrose, 50 mM Tris/Cl−, 0.2 mM EDTA, pH 8.0). The residual NADH oxidase activity was initiated by the addition of 0.1 mM NADH and gramicidin D (0.05 μg/mL). Potassium succinate (2.5 mM) was present during incubation with NADH-OH in the sample marked as □ (reduced complex I), and 2.5 mM potassium malonate was present in the NADH oxidase assay. The continuous lines are the computer-generated curves for the simple bimolecular irreversible enzyme–inhibitor interaction with the second-order rate constant of 1.6 × 108 (●, ○, ■) and 1.5 × 107 (□, reduced complex I) M−1 min−1. (B) Pseudo-first-order rate logarithmic anamorphosis of the data shown in (A). The inset shows the linear dependence of the apparent first-order rate constant on the concentration of NADH-OH.