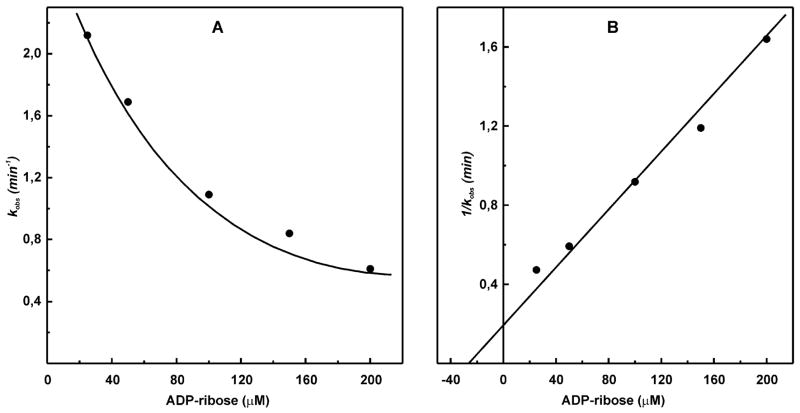
Figure 4.
Effect of ADP-ribose on irreversible inhibition of complex I by NADH-OH. (A) SMPs (25 μg/mL) were incubated for 1 min in the standard reaction mixture containing 28 nM NADH-OH and ADP-ribose (concentrations are indicated on the abscissa). The residual NADH oxidase activities (100 μM NADH and 0.05 μg/mL gramicidin D) were determined. The pseudo-first-order rate constants for NADH-OH-induced inhibition were calculated as kobs = ln(v0/v t) and plotted as a function of ADP-ribose concentration. v0 corresponds to the rate of NADH oxidation in the absence of NADH-OH, and vt is the residual activity after 1 min of incubation with NADH-OH. (B) Secondary plot of 1/kobs versus ADP-ribose concentration used to determine KD for ADP-ribose.