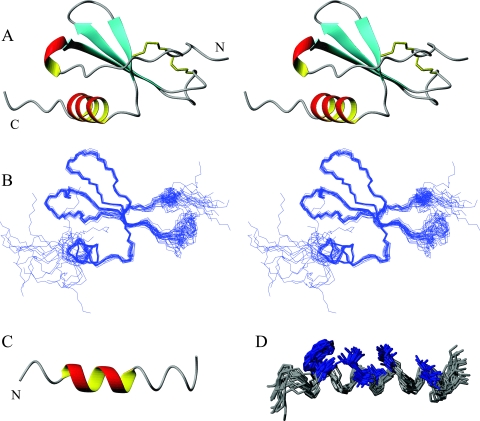
FIG. 2.
(A) Stereo ribbon diagram of the water-refined, lowest-energy structure of MIP-3α. The two disulfide bonds are represented in yellow, and the N and the C termini are labeled. (B) Stereo diagram showing an overlay of the protein backbone of the 20 lowest-energy structures. The structures were superimposed over the backbone atoms of residues 6 to 65 and are shown in the same orientation as the stereo ribbon diagrams in panel A. The two termini and the 30s loop (on right-hand side) display the largest amount of disorder. (C) Ribbon diagram of the lowest-energy structure of the MIP-3α C-terminal peptide. (D) Representation of the 20 lowest-energy conformers of the MIP-3α peptide fit to the backbone atoms of residues 4 to 18. The central region is well defined, while the termini are more disordered. Hydrophobic residues Trp, Val, Ile, and Leu (highlighted in blue) assemble on one side of the peptide to form its hydrophobic face. The figure was prepared with the MOLMOL program (39).