Abstract
The response of microbial functional diversity as well as its resistance to stress or disturbances caused by the introduction of an exotic tree species, Acacia holosericea, ectomycorrhized or not with Pisolithus albus, was examined. The results show that this ectomycorrhizal fungus promotes drastically the growth of this fast-growing tree species in field conditions after 7 years of plantation. Compared to the crop soil surrounding the A. holosericea plantation, this exotic tree species, associated or not with the ectomycorrhizal symbiont, induced strong modifications in soil microbial functionalities (assessed by measuring the patterns of in situ catabolic potential of microbial communities) and reduced soil resistance in response to increasing stress or disturbance (salinity, temperature, and freeze-thaw and wet-dry cycles). In addition, A. holosericea strongly modified the structure of arbuscular mycorrhizal fungus communities. These results show clearly that exotic plants may be responsible for important changes in soil microbiota affecting the structure and functions of microbial communities.
Forest loss and degradation through human disturbance as well as deterioration of land productivity is a major problem in large areas of arid and semiarid environments. Degraded soils are characterized by loss or disturbance of the vegetation cover, increased soil erosion, decreased in-water infiltration, loss of available nutrients and organic matter, loss of microbial propagules, and/or diminution in microbial activity (25, 35, 44). Restoration of ecosystem health and productivity was traditionally achieved via abandonment of land and subsequent natural forest succession. In recent decades, management options for acceleration of the recovery and restoration of the productivity and biodiversity of disturbed ecosystems have been considered, since fallow periods have been shortened or eliminated due to increased anthropogenic pressure and agriculture intensification (49). Concerning the techniques used for rehabilitating degraded areas, there is increasing evidence that forest plantations can play a key role in ecosystem rehabilitation or restoration (52). Among candidate plantation species, fast-growing leguminous tree species (e.g., Australian Acacia species) should have preference.
Acacia is the largest mimosoid genus, with 1,200 species (53), and their presence in savannas and arid regions of Australia, Africa, India, and the Americas has been frequently recorded. These multipurpose trees are useful for revegetation of degraded ecosystems that have low availability of nitrogen and phosphorus (4, 6, 13, 14, 55) because of their ability to form symbiotic associations with both rhizobial bacteria and mycorrhizal fungi. These bacterial and fungal symbionts are known to be key components of natural systems (8, 64, 65, 66), since they are involved in governing the cycles of major plant nutrients and in sustaining the vegetation cover in natural habitats (63). However, the success of revegetation programs may be limited by the low density of mycorrhizal propagules generally observed in degraded soils in semiarid and arid ecosystems (25). Hence, it may be necessary to reinforce or replace the native inoculum potential of mycorrhizal fungi by appropriate inoculation technologies (29, 30). Fast-growing leguminous trees belonging to the Acacia genus, brought from Australia and introduced to Western Africa, appear to be well adapted to sahelian and Sahelo-Sudanian climatic conditions (11, 57). In addition to rhizobial symbiosis, this tree species can form arbuscular mycorrhiza (AM) and/or ectomycorrhiza (19, 31). As with many N2-fixing trees and shrubs, Australian Acacia species are especially dependent on mycorrhizas to absorb mineral nutrients required for plant growth and efficient N2 fixation. It has been previously demonstrated that controlled mycorrhizal inoculation could improve the development of these tree species in glasshouse conditions (24, 26, 27) and also after outplanting into the field (10, 29, 30). All these studies have been mainly focused on the biomass production of Acacia plantations, and little is known about the influence of mycorrhizal inoculation on the functional diversity of native microbial communities involved in soil functioning, especially when these soils are planted with exotic tree species.
The present study addressed the following questions: (i) does the microbial functional diversity of a site planted by exotic tree species differ from that of an adjacent unplanted site, and if so, (ii) does ectomycorrhizal inoculation influence this microbial response? To answer these questions, the effect of the presence of an ectomycorrhizal fungus, Pisolithus albus IR100, was determined by examining the development of an Australian acacia, A. holosericea, in a field plantation in a dry tropical environment. In addition, modifications induced by this exotic tree species, associated or not with P. albus IR100 treatment, have been measured in microbial functional capabilities and in the structure of native arbuscular mycorrhizal fungus (AMF) communities, an essential component of sustainable soil-plant systems (39).
MATERIALS AND METHODS
(i) Plant material and ectomycorrhizal inoculation.
Seeds of A. holosericea, ex G. Don, provenance Bel Air (Dakar, Senegal), were surface sterilized with concentrated 36 N sulfuric acid for 60 min. They were then rinsed for 12 h in sterile distilled water and transferred aseptically to petri dishes filled with 1% (wt/vol) agar/water medium. After 8 days of incubation at 25°C in the dark, the germinating seeds were used when rootlets were 1 to 2 cm long.
The ectomycorrhizal fungus Pisolithus albus IR100 was routinely maintained on modified Melin-Norkrans (MMN) agar medium at 25°C (48). Glass jars (1 liter) were filled with 600 ml of a mixture of vermiculite and peat moss (4/1 [vol/vol]) and autoclaved (120°C for 20 min). The substrate was then moistened to field capacity with 300 ml of liquid MMN medium. The jars were sealed with cotton floats and autoclaved at 120°C for 20 min. After cooling, the substrate was inoculated with fungal plugs taken from the margin of the fungal colonies and incubated for 6 weeks at 28°C in the dark (23).
Acacia seedlings were grown in 1-liter pots filled with an autoclaved sandy soil (120°C for 40 min) collected from a stand of A. holosericea trees located east of Dakar (Senegal). After sampling, the soil was crushed, passed through a 2-mm-pore-size sieve, and autoclaved for 40 min at 120°C to eliminate the native microbiota. After autoclaving, its physicochemical characteristics were as follows: H2O pH, 5.3; clay, 3.6%, fine silt, 0.0%; coarse silt, 0.8%; fine sand, 55.5%; coarse sand, 39.4%; carbon, 0.17%; total nitrogen, 0.02%; soluble phosphorus, 4.8 mg kg−1; total phosphorus, 39 mg kg−1. The ectomycorrhizal inoculation was performed by mixing the fungal inoculum with the soil (10/1 [vol/vol]). The control treatment (without fungus) included an autoclaved mixture of vermiculite and peat moss moistened in MMN medium at the same rate. The pots were arranged in a randomized complete block design. The plants were grown in a glasshouse under natural light conditions (approximately 12 h of daylight, mean daily temperature 30°C) and watered daily with tap water.
After 4 months of culturing, 10 plants were randomly chosen from each treatment. They were uprooted and their root systems gently washed. The oven dry weight (1 week at 65°C) of the shoot was measured. Then, the root systems were cut into 1-cm-long root pieces and mixed. The percentage of ectomycorrhizal short roots (number of ectomycorrhizal short roots/total number of short roots) was determined using a random sample of at least 100 short roots per treatment under a stereomicroscope (magnification ×40). Some nodules were observed along the root systems despite disinfection of the soil and the seed surface. Root nodules were counted and their dry weight (after 65°C for 1 week) determined. The dry weight of roots (after 1 week at 65°C) was then measured.
(ii) Experimental design, tree growth measures, and soil sampling.
The study site was located in Senegal at Ngane village (17°50′W to 14°10′N) (15 km at the west of Kaolack) on a ferrugineous soil (29, 30). The climate is Sahelo-Sudanian and tropically dry, with an average annual rainfall of 700 mm and a mean annual temperature of 25°C. Two seasons can be distinguished: (a) the long dry season from October to June and (b) the rainy season from July to September. The physicochemical characteristics of the soil were as follows: H2O pH, 5.2; clay, 8.7%; fine silt, 6.5%; coarse silt, 17.6%; fine sand, 40.8%; coarse sand, 25.6%; carbon, 0.4%; total nitrogen, 0.033%; soluble phosphorus, 4.6 mg kg−1; and total phosphorus, 54.7 mg kg−1.
The field trial had a randomized block design with one factor and three replication blocks. The factor was the direct ectomycorrhizal inoculation of A. holosericea seedlings with P. albus IR100 or not (control) in the glasshouse. The plot size was 6 m by 6 m. Each plot was separated from the other plots by a nonplanted area 6 m wide. In July 2000, A. holosericea seedlings were planted in individual holes (20 cm diameter and 30 cm depth) at 3 m apart in an area of 900 m2. They were at least 27 seedlings per treatment and replication (nine plants and two treatments in each block). The dead plants were replaced in each treatment block during the first months of plantation. After 7 years of plantation, the stem diameter of each tree was measured at 0.1 m above the soil. Then, phyllode, small branch, wood, and litter biomasses were evaluated according to allometric relations previously established (30).
A transect was marked out along the diagonal of each plot with two sets of soil samples (four samples per set that were pooled together). In addition, six soil samples were taken from the surrounding cultivated area (millet plantation) of the A. holosericea plantation. These soil samples (250 cm3 each) were collected during the wet season from the 0 to 10 cm layer and stored in sealed plastic bags with field moisture content at 4°C for further measurements. All soil samples were characterized by measuring total C, total N, total P, and soluble P levels (50) in the Laboratoire des Moyens Analytiques laboratory (Certifié International Standard for Organization 9001, version 2000, Dakar, Senegal; US Imago [Unité de Service Instrumentations, Moyens Analytiques, Observatoires en Geophysique at Océanographie], Institut de Recherche pour le Développement [www.lama.ird.sn]).
(iii) Soil characteristics. (a) Measurement of microbial functional diversity.
Microbial functional diversity of soil treatments was assessed by measuring the patterns of in situ catabolic potential of microbial communities (15). This method is based on the measurement of short-term respiration responses of soils amended with a range of simple organic compounds (15). One gram of equivalent dry soil was moistened with each of the 33 (15) substrates suspended in 2-ml sterile distilled water in 10 ml bottles (68). CO2 production from basal respiratory activity in the soil samples was measured by adding 2 ml sterile distilled water to 1 g equivalent dry weight of soil. The bottles were sealed with a Vacutainer stopper and incubated at 28°C for 4 h in the dark. The amount of respired CO2 in the headspace of each bottle was determined by taking a 1-ml syringe sample. The CO2 concentration was analyzed for each sample by using an infrared gas analyzer (Polytron IR CO2; Dräger) in combination with a thermal flow meter (38). Carbon dioxide measurements were subtracted from the CO2 basal production measurements and were expressed as micrograms of CO2 per gram of soil per hour. Among the 33 substrates, there were nine amino acids (l-arginine, l-serine, l-glutamic acid, l-phenylalanine, l-asparagine, l-lysine, l-cysteine, l-tyrosine, and l-histidine), two amines (d-glucosamine and l-glutamine), two amides (N-methyl-d-glucamine and l-succinamide), three carbohydrates (d-mannose, d-sucrose, and d-glucose), and 17 carboxylic acids (α-ketobutyric acid, α-ketoglutaric acid, fumaric acid, oxalic acid, tartaric acid, gluconic acid, ascorbic acid, dl-malic acid, malonic acid, quinic acid, 3-OH-butyric acid, formic acid, gallic acid, succinic acid, trisodium citric acid, uric acid, and citric acid). The amines and amino acids were added at 10 mM, whereas the carbohydrates were added at 75 mM and the carboxylic acids at 100 mM (16). Catabolic evenness (a measure of relative variability in the catabolic functions) was calculated using the Simpson-Yule index E = 1/p2i, with pi = (respiration response to individual substrates)/(total respiration activity induced by all substrates for a soil treatment) (47). Microbial biomass C levels calculated using the substrate-induced respiration method as described by Sparling (60). One gram of oven-dry weight of soil was suspended in 2 ml of a 75 mM glucose solution in 10 ml bottles sealed with a Vacutainer stopper and incubated at 25°C for 4 h. After correction for CO2 produced in bottles with only deionized water added, the microbial biomass C levels were calculated as follows: (micrograms of C per gram of soil) = 50.4 × respiration rate (micrograms of C per gram of soil per hour).
(b) Assessment of soil microbial resistance to stress and disturbance.
Each soil sample, maintained at field moisture content (50% of maximum water holding capacity), was subjected to one stress and three disturbance treatments according to the methods described by Degens et al. (18). The stress treatment consisted of increasing soil salinity. The disturbance treatments consisted of increasing soil temperature and consecutive wet-dry and freeze-thaw cycles. Control treatments were performed by a continuous incubation at 25°C in the dark to verify whether the incubation period imposed to soil samples in the wet-dry and freeze-thaw treatments (24 days) had any effects on microbial activity.
Three levels of the salinity stress treatment were obtained using a solution of NaCl to modify the soil salinity, which was increased from an electrical conductivity level of 0.04 mS cm−1 (control without NaCl addition) to 0.3, 0.7, and 1.1 mS cm−1.
For temperature disturbance treatment, soil samples were incubated at 25°C (control), 37°C, 45°C, and 50°C in the dark for 1 week.
Each wet-dry cycle consisted of air drying at 25°C for 24 h followed by a rapid rewetting to initial field moisture content and incubation for 48 h in the dark at 25°C. One, two, four, six, and eight successive wet-dry cycles were imposed on soil samples. Freeze-thaw cycles consisted of freezing at −20°C for 24 h followed by thawing and incubation at 25°C for 48 h. Soil samples were subjected to one, two, four, six, and eight successive freeze-thaw cycles.
There were three replicates per stress level. After each stress or disturbance treatment, soil samples were kept at 25°C in sealed plastic bags for 2 weeks to permit equilibration of the microbial communities and decomposition of organic C from microorganisms destroyed by the treatments (18).
(c) Total soil microbial activity (FDA).
After each stress or disturbance treatment, total microbial activity in soil samples was estimated using a fluorescein diacetate (3′,6′-diacetylfluorescein [FDA]) hydrolysis assay (58). FDA (Sigma-Aldrich Chemie, France) was dissolved in acetone and stored as a stock solution (5 mg ml−1) at −20°C. Soil samples (1 g equivalent dry weight) were suspended in 200 μl FDA and 15 ml of sterile 60 mM sodium phosphate buffer (pH 7.6). The mixture was incubated at 25°C for 1 h on a rotary shaker. Then, the FDA hydrolysis reaction was stopped by adding 750 μl acetone. Soil suspensions were centrifuged (2,400 × g for 10 min), and the supernatant was sampled and passed through a 45-μm-pore-size filter. Then, the absorbance readings were taken at 490 nm. Three replicates were prepared for each treatment, and a fourth received 15 ml of buffer without substrate; this served as a control to correct for background. A standard fluorescein concentration curve, ranging from 0 to 0.5 mg liter−1, was prepared fresh using the stock solution of fluorescein diacetate in sodium phosphate buffer. The rate of fluorescein diacetate hydrolysis was calculated (in micrograms of product corrected for background fluorescence per hour per gram of soil) to determine total microbial activity for each soil origin.
(d) Description of the structure of AMF communities.
Spores of AM fungi were extracted from soil samples by wet sieving and decanting followed by sucrose centrifugation (59). Then, the supernatant was poured through a 50-μm-pore-size sieve and rinsed with tap water. Spores were counted using a stereomicroscope and grouped according to morphological characteristics. The uniformity of morphological groups was confirmed under the optical microscope, and the different morphotypes were identified with respect to genus. Spore identification was assessed, mainly using spore size and color, wall structure, and hyphal attachment (67) (http://www.invam.caf.wvu.edu/). Mycorrhizal fungal spore diversity was calculated using the Simpson-Yule diversity index (43).
(iv) Statistical analysis.
Data were analyzed using one-way analysis of variance. Means were compared using the Newman-Keuls test (P < 0.05). The distribution of AMF species was compared between sets of soil origins using two-by-two contingency tables and χ2 tests and Yates correction for small numbers.
Between-group analysis (BGA) (12, 22) was used to analyze the relationships between substrate-induced responses (SIRs) and the three soil origins: crop soil (i.e., soil samples collected from the crop soil surrounding the A. holosericea plantation), soil samples collected under uninoculated A. holosericea trees, and soil samples collected under A. holosericea trees inoculated with P. albus IR100. BGA is a multivariate analysis technique derived from principal components analysis (PCA). The aim of PCA is to summarize a data table by searching orthogonal axes to determine the projection of the sampling points (rows of the table) that has the highest possible variance. This characteristic ensures that the associated graphs (principal component maps) will best represent the initial data. These principal components have the property of having the highest possible correlation with the original variables (as shown in the columns of the data table).
From a theoretical point of view, BGA is a particular case of PCA with respect to instrumental variables (45, 54) in which the instrumental variable table is reduced to just one qualitative variable. This variable defines groups of rows in the data table, and BGA consists of the PCA of the table of the means by groups. This table has a number of rows equal to the number of groups and the same number of columns as the original table. The objective of this analysis is to separate the groups. This is also the aim of discriminant analysis (also called canonical variate analysis), but while discriminant analysis is limited to tables that have a high number of samples compared to the number of variables, BGA can be used even when the number of rows is less than the number of variables, as in the present study (33 SIRs versus nine soil samples). BGA can thus be considered a robust alternative to discriminant analysis in experiments with a low number of samples. A Monte-Carlo test (permutation test) was used to check the significance of the differences between groups. Computations and graphical displays were realized with the free ADE-4 software (62) available on the Internet at http://pbil.univ-lyon1.fr/ADE-4/.
RESULTS
Plant growth.
After 4 months of culture growth under glasshouse conditions, ectomycorrhizal inoculation significantly enhanced shoot and root growth (Table 1). Compared with the control results, the levels of shoot and root growth of P. albus IR100 plants were stimulated to 2.4-fold and 2.3-fold increases, respectively (Table 1). Although soil was autoclaved and seeds of A. holosericea were surface sterilized, some rhizobial nodules have been observed on the root systems. It was assumed that the tap water used during this experiment contained contaminant rhizobia. The number of nodules per plant and the total nodule biomass per plant were significantly increased in the P. albus IR100 treatment compared to the control results (Table 1).
TABLE 1.
Effect of P. albus inoculation on the growth of A. holosericea seedlings and on nitrogen fixative symbiosis after 4 months of culture growth under glasshouse conditionsa
| Treatment | Shoot biomass (mg dry wt) | Root biomass (mg dry wt) | No. of nodules per plant | Total nodule biomass per plant (mg dry wt) | Ectomycorrhizal colonization index (%) |
|---|---|---|---|---|---|
| Control (no inoculation) | 650a | 351a | 8.6a | 2.8a | |
| P. albus IR100 inoculation | 1,545b | 795b | 12.3b | 10.5b | 30.5 |
Data followed by the same letter in each column are not significantly different according to results of the Newman-Keuls test (P < 0.05).
Seven years after tree transplantation, the height, diameter, and phyllode, small branch, and wood biomasses as well as litter biomass in the ectomycorrhizal treatment samples were significantly higher than in the control samples (Table 2). For instance, litter biomass and wood biomass were increased 3.1-fold and 5.6-fold in the P. albus IR100 treatment samples, respectively (Table 2).
TABLE 2.
Effect of P. albus IR100 inoculation on the growth of A. holosericea after 7 years of plantation in the fielda
| Treatment | Height (m) | Stem diam (cm) | Leaf biomass (kg per tree) | Small branch biomass (kg per tree) | Wood biomass (kg per tree) | Total aboveground biomass (kg per tree) | Litter biomass (kg m−2) |
|---|---|---|---|---|---|---|---|
| Control (no inoculation) | 4.84a | 11.8a | 19.1a | 16.9a | 27.1a | 63.1a | 1.98a |
| P. albus IR100 inoculation | 6.43b | 23.2b | 82.6b | 75.1b | 153.2b | 310.9b | 6.19b |
Data followed by the same letter in each column are not significantly different according to results of the Newman-Keuls test (P < 0.05).
Chemical and microbial characteristics of soils.
Soil nitrogen, carbon, and soluble phosphorus contents were significantly higher in the P. albus IR100 treatment samples, whereas under inoculated A. holosericea trees, the microbial biomass was significantly lower than the level seen with the other treatments (soil collected from the uninoculated A. holosericea treatment and outside the tree plantation from the cultivated area) (Table 3). Soil total phosphorus contents were significantly different for soil sampled from the cultivated field compared to the results seen with the P. albus IR100 treatment (Table 3).
TABLE 3.
Microbial biomass and chemical characteristics of the soils sampled inside the A. holosericea plantation from each treatment (uninoculated plots and P. albus-inoculated plots) and outside the A. holosericea plantation (crop soil)a
| Soil origin | Total nitrogen (%) | Total carbon (%) | Total phosphorus (mg kg−1) | Soluble phosphorus (mg kg−1) | Microbial biomass (μg C g−1 soil) |
|---|---|---|---|---|---|
| Crop soil | 0.032a | 0.398a | 67.0a | 4.6a | 278.7b |
| Plantation with uninoculated trees | 0.024a | 0.338a | 73.2ab | 4.9a | 227.5a |
| Plantation with P. albus IR100-inoculated trees | 0.046a | 0.600b | 84.2b | 7.1b | 231.2a |
Data followed by the same letter in each column are not significantly different according to results of the Newman-Keuls test (P < 0.05).
The catabolic evenness of the crop soil was significantly higher than those recorded in the other treatments (Table 4). The highest average respiration SIRs to amides and carbohydrates were recorded with the crop soil (Table 4), whereas the lowest SIR to amino acids was recorded with the uninoculated A. holosericea treatment (Table 4). Significant differences were found in the average SIRs to amines and carboxylic acids between the crop soil treatment and uninoculated A. holosericea treatment results, but for amino acids the highest SIR was found in the crop soil and for carboxylic acids in the uninoculated A. holosericea treatment (Table 4).
TABLE 4.
Catabolic evenness and average SIR with each substrate group (carboxylic acids, amino acids, amides and carbohydrates) in the soil treatmentsa
| Soil origin | Catabolic evenness | Avg SIR (μg CO2 g−1 soil h−1)
|
||||
|---|---|---|---|---|---|---|
| Amino acids | Amides | Amines | Carbohydrates | Carboxylic acids | ||
| Crop soil | 16.5b | 8.7b | 9.3b | 9.8b | 18.5b | 14.3a |
| Plantation with uninoculated trees | 9.8a | 6.3a | 4.3a | 7.9a | 10.9a | 16.6b |
| Plantation with P. albus IR100-inoculated trees | 9.5a | 7.9b | 3.6a | 8.3ab | 12.9a | 14.8ab |
Data followed by the same letter in each column are not significantly different according to results of the Newman-Keuls test (P < 0.05).
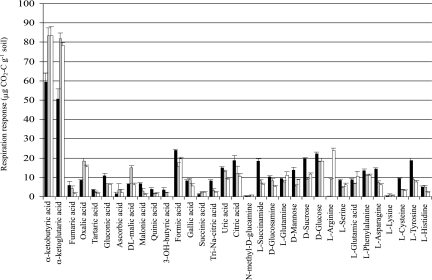
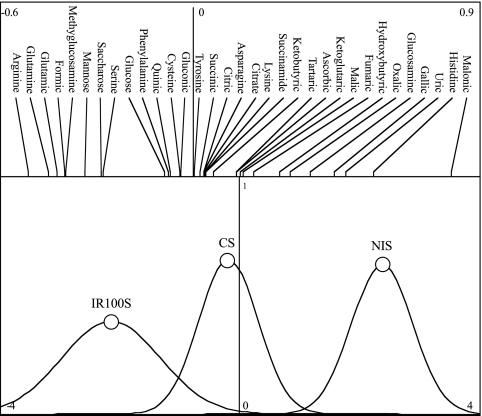
The three treatments gave very different SIR profiles (Fig. 1 and Fig. 2). The highest SIRs have been obtained with α-ketobutyric and α-ketoglutaric acids (Fig. 1). The permutation test of BGA showed that microbial functionalities were very different according to the soil treatment (P < 0.001) (Fig. 2). The three treatments were very well separated on the BGA second axes (Fig. 2). The substrates preferentially used in samples collected from the field were l-asparagine, succinamide, cysteine, tyrosine, and trisodium citrate (Fig. 2). In contrast, the substrate preferentially used in the soil collected under uninoculated A. holosericea trees was malonic acid whereas it was l-arginine in the soil sampled under A. holosericea trees inoculated with P. albus IR100 (Fig. 2).
FIG. 1.
Catabolic response profiles for each soil origin. Error bars represent standard errors (n = 6). Black bars, crop soil; gray bars, plantation soil with uninoculated trees; white bars, plantation soil with P. albus IR100-inoculated trees.
FIG. 2.
Graphical display of the second BGA axis showing the SIRs with respect to the soil treatments. Only the second axis is used here, as the first axis merely separated the crop soil samples. The upper part of the figure shows the scores of the 33 substrates on the second BGA axis. In the lower part, the three Gauss curves represent the mean and variance of the scores of the nine soil samples (three repetitions for three treatments) on the second BGA axis. CS, crop soil; NIS, soil of plantation with uninoculated trees; IR100S, soil of plantation with P. albus IR100-inoculated trees. Substrates represented by lines curved in the same direction as corresponding Gauss curves tended to be used more in the corresponding soil samples.
Total microbial activities in stressed and disturbed soils.
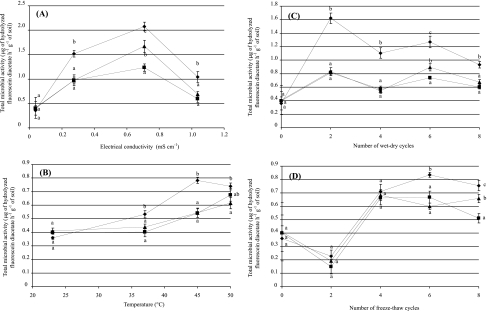
Before any stresses or disturbances were imposed to the soil treatments, their total microbial activities were not significantly different (mean, 0.389 μg of hydrolyzed fluorescein diacetate h−1 g−1 of soil). In addition, the longer incubation period required in imposing wet-dry and freeze-thaw treatments did not significantly affect the total microbial activities of soil samples. The effects of each stress and disturbance regimen on total microbial activities caused significant differences between soils with the highest catabolic evenness (crop soil) and those with the lowest catabolic evenness (soil sampled from A. holosericea plantation).
Increasing the salt stress caused a higher increase in the total microbial activities of crop soil up to 0.7 mS cm−1; after that, microbial activities decreased (Fig. 3A). At 0.7 mS cm−1, microbial activities ranged among the treatments as follows: crop field→plantation soil with P. albus IR100-inoculated trees→plantation with uninoculated trees and, at 1.0 mS cm−1, crop field→A. holosericea plantation (with or without P. albus inoculation).
FIG. 3.
Responses of total microbial activity to increased levels of stress induced by increases of NaCl (A) or disturbance by increases in temperature (B) or by wet-dry cycles (C) or freeze-thaw cycles (D). •, crop soil; ▪, soil of plantation with uninoculated trees; □, plantation soil with P. albus IR100-inoculated trees. Error bars represent standard errors (n = 6). For each level of stress or disturbance, data indexed by the same letter are not significantly different according to results of the Newman-Keuls test (P < 0.05).
Increasing soil temperature induced similar changes in each soil (Fig. 3B) but with a higher response seen with the crop soil (Fig. 3B). There was an increase of soil microbial activities for each treatment with all the temperatures imposed on the soil samples and a slight decrease for the crop soil after 45°C treatment (Fig. 3B).
Responses to wet-dry cycles were significantly higher with the crop soil than with the other soil treatments (Fig. 3C). Microbial activities increased up to two cycles and then decreased (Fig. 3C). At six cycles, the microbial activity was higher in the soil collected under inoculated A. holosericea trees than in that sampled under uninoculated A. holosericea trees.
Freeze-thaw cycles had effects opposite those recorded when the soils were imposed to wet-dry cycles (Fig. 3D). Microbial activities decreased up to two cycles and afterward decreased slightly (Fig. 3C). At eight cycles, the microbial activity was higher in the soil collected under inoculated A. holosericea trees than in that sampled under uninoculated A. holosericea trees (Fig. 3D).
AMF communities.
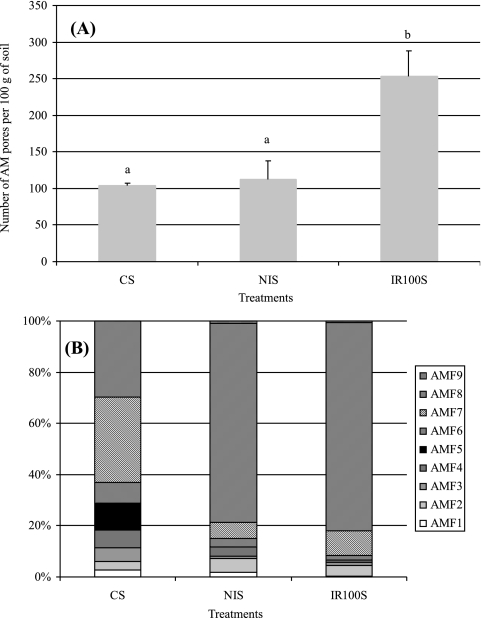
The number of spores was significantly higher in the soil collected under inoculated A. holosericea trees (253 spores per 100 g of soil) than in both of the other soil categories (104 spores and 112 spores per 100 g of soil in the crop soil and in the soil collected under uninoculated A. holosericea trees, respectively). Nine AM species were detected in the soils: Scutellospora gregaria; S. verrucosa; Scutellospora sp. strain 1; Gigaspora sp. strain 1; Gigaspora sp. strain 2; Glomus rubiforme; G. aggregatum; Glomus sp. strain 1; and Glomus sp. strain 2 (Fig. 4). Simpson indices (SI) ranged as follows: crop soil (7.66 ± 1.1)→soils from A. holosericea plantation (inoculated, SI = 4.7 ± 0.2; uninoculated, SI = 5.2 ± 0.4). Spores of Glomus sp. strain 1 were mainly recorded in the soils collected from the A. holosericea plantation (Fig. 4). The distributions of AM species within soil origins were significantly different between the crop soil and the soil collected under uninoculated A. holosericea trees (χ2 = 63.9 [P < 0.0001]) and between the crop soil and the soil collected under inoculated A. holosericea trees (χ2 = 106.6 [P < 0.0001]). No significant differences were recorded between the AM species distributions of AM communities under inoculated and uninoculated trees.
FIG. 4.
Distribution in each soil origins of arbuscular mycorrhizal fungus species. CS, crop soil; NIS, soil of plantation with uninoculated trees; IR100S, soil of plantation with P. albus IR100-inoculated trees. AMF1, Scutellospora gregaria; AMF2, S. verrucosa; AMF3, Scutellospora sp. strain 1; AMF4, Gigaspora sp. strain 1; AMF5, Gigaspora sp. strain 2; AMF6, Glomus rubiforme; AMF7, G. aggregatum; AMF8, Glomus sp. strain 1; AMF9, Glomus sp. strain 2.
DISCUSSION
This study clearly shows that (i) A. holosericea growth is greatly enhanced by the presence of the ectomycorrhizal fungus P. albus IR100 after 7 years of plantation; (ii) ectomycorrhizal inoculation induces significant changes in the functions of soil microbial communities; and (iii) the introduction of an exotic tree species significantly alters soil microbial functionalities (i.e., resistance to stress and disturbance) and the structure of AMF communities.
Inoculation with P. albus IR100 was highly beneficial to the growth of A. holosericea in the disinfected sandy soil, and this stimulating effect was retained during 7 years of culture growth in field conditions. This result is in accordance with other studies in which it was demonstrated that this fungal isolate was very efficient with respect to enhancement of the growth of Australian Acacia species (1, 27, 29, 30) and Casuarina species (28). Under field conditions, this positive effect of fungal inoculation on A. holosericea growth was previously recorded in the same region but after shorter periods (18 or 30 months of plantation) (30). The measurements found in the present study show that the fungal effect on the plant growth can be kept for a longer duration. Some Pisolithus fruiting bodies have been detected under inoculated A. holosericea trees (R. Duponnois, unpublished data) after 3 to 5 years of plantation, confirming the development of the fungal inoculant in the soil. After 5 years of plantation, Pisolithus fruiting bodies disappeared (Duponnois, unpublished data). Quantitative and qualitative differences in the occurrences of carpophores of ectomycorrhizal fungi between stands of different ages are well known (21). According to Dighton and Mason (21), the number of fruiting bodies in the stand declined at a later stage.
Total nitrogen, carbon, and soluble phosphorus contents were significantly higher in soil collected under ectomycorrhized A. holosericea trees, whereas no significant differences were recorded for the C/N ratios among soil treatments. The effect of ectomycorrhizal inoculation on soil carbon content could be easily explained, as a greater biomass of litter was measured under ectomycorrhized A. holosericea trees. Decomposition results in the transformation of organic forms of nitrogen, phosphorus, and sulfur, which are found in litter, into mineral forms that are readily absorbed by plants (2). Numerous studies of the effects of roots on decomposition in the soil have produced contradictory results. It has been found that in forest soils, roots and ectomycorrhizal fungi significantly retard decomposition rates of litter decomposition through the “Gadgil effect” (33, 34). In contrast, roots stimulate greater activity of the soil biota in soils with low total C and N contents and, in turn, contribute to faster litter decomposition and nutrient release (69). In the present study, although roots of A. holosericea (inoculated or not with P. albus IR100) decreased microbial biomass, soil N and P levels were enhanced in the P. albus treatment. Olsson et al. (51) have found that the presence of ectomycorrhizal mycelia reduced bacterial activity in a sandy soil, whereas it has been previously demonstrated that ectomycorrhizal symbiosis had a selective pressure on bacterial communities by promoting the development of bacterial strains potentially beneficial to the symbiosis and to the plant (i.e., organic phosphate-solubilizing bacteria) (32). It is generally admitted that ectomycorrhizal fungi have a reduced ability to decompose complex organic compounds of carbon (9), but the impact of ectomycorrhizal symbiosis on soil microbiota could promote the process of decomposition of organic N and P compounds, leading to higher soil P and N contents. Changes in the catabolic response profiles also revealed this mycorrhizosphere effect (46). A higher SIR with l-arginine was recorded from the soil collected under inoculated A. holosericea trees. Duponnois et al. (29) found that ectomycorrhizal inoculation significantly enhanced root growth of this Australian acacia in field conditions. Since l-arginine has been detected in root exudates (5), a higher amount of this amino acid could exert a selective influence on soil microbial communities through a multiplication of arginine-catabolizing microorganisms while inducing a higher SIR.
Compared to the crop soil, the A. holosericea plantation induced strong modifications in soil microbial functionalities that reduced soil resistance to increasing stress or disturbance. In the present study, the catabolic evenness of the crop soil was 16.5, which was in accordance with previous studies in which values of catabolic evenness for soils under cropping conditions ranged from 16.4 to 19.6 (17). After 7 years of plantation, A. holosericea trees (inoculated or not with P. albus IR100) had significantly decreased levels of soil catabolic evenness to 9.8 (soil of plantation with uninoculated trees) and 9.5 (soil of plantation with P. albus IR100-inoculated trees), values that are rather low compared to the values reported for the literature (17) but that have already been reported for a glasshouse experiment with Eucalyptus camaldulensis (40). Stevenson et al. (61) compared the catabolic respiration responses of microbial communities from pastures and forest soils. They found that pasture soil communities had significant higher responses to carbohydrate and amino acid substrates and significant lower relative responses to carboxylic acid substrates than microbial communities from forest soils. In the present study, taking into account the measurements from the crop soil and from the soil of plantation with uninoculated trees, our results are in accordance with those of Stevenson et al. (61) for the SIRs seen with amino acids, carbohydrates (higher levels in the crop soil), and carboxylic acids (higher levels in the tree plantation soil). In addition, our results confirm the differential responses of glucose and α-ketobutyric acid between crop and tree plantation (61). Ectomycorrhizal inoculation slightly modified the SIR differences between the two soil origins with respect to amino acids, amines, and carboxylic acids. It has been previously demonstrated that mycorrhizal symbiosis can counterbalance the influence of exotic tree species (E. camaldulensis) on the functioning of soil microbial communities in a sahelian soil (40).
Degens et al. (18) showed that soils with reduced catabolic evenness are less resistance to stress and disturbance. These results are consistent with those recorded in the present study. Soil collected outside the A. holosericea plantation (highest catabolic evenness) was more resistant to stress and disturbance than the soils collected inside the A. holosericea plantation (lowest catabolic evenness). Microbial activity showed a classical “hump-back” pattern in response to increasing stress or disturbance. This effect (predicted by the intermediate disturbance hypothesis) has been frequently reported for plant communities (3) but more rarely for soil microbial communities (18, 20). According to this hypothesis, it is assumed that under conditions of minimal stress or disturbance microbial diversity is poor and that under conditions of greater stress and disturbance this microbial diversity increases until stress or disturbance reaches levels that permit the growth of only a few species.
Numerous studies have clearly shown that structurally and functionally distinct microbial communities develop under different plant species (36, 37, 42). Several well-documented studies have shown that exotic plant species can significantly alter soil biological and chemical characteristics (40, 41, 42). In particular, it has been found that exotic plants could alter the structure of AM fungus communities (40) and disrupt mutualistic associations between existing ecological associations within native communities (7, 56). It has been suggested that introduced plant species had a selective positive influence on some AM species within the AM fungus native communities (41). In the present study, a similar process was found, as A. holosericea drastically promoted the multiplication of one fungal species (Glomus sp. strain 1) and consequently altered the species evenness of AM communities.
While the effects of microbial communities (structure and functions) on plant community diversity and soil biofunctioning have attracted considerable attention, our results clearly show that exotic plants may be directly responsible for important changes in soil microbiota affecting the structure and functions of microbial communities.
Acknowledgments
We are very grateful to M. Diouf, who has looked after the plantation for 7 years.
Footnotes
Published ahead of print on 18 January 2008.
REFERENCES
- 1.André, S., A. Galiana, C. Le Roux, Y. Prin, M. Neyra, and R. Duponnois. 2005. Ectomycorrhizal symbiosis enhanced the efficiency of inoculation with two Bradyrhizobium strains and Acacia holosericea growth. Mycorrhiza 15:357-364. [DOI] [PubMed] [Google Scholar]
- 2.Attiwill, P. M., and M. A. Adams. 1993. Nutrient cycling in forests. New Phytol. 124:561-582. [DOI] [PubMed] [Google Scholar]
- 3.Austin, M. P. 1987. Models for the analyses of species' response to environmental gradients. Vegetatio 69:35-45. [Google Scholar]
- 4.Bethlenfalvay, G. J., S. Dakessian, and R. S. Pacovsky. 1984. Mycorrhizae in a southern California desert: ecological implications. Can. J. Bot. 62:519-527. [Google Scholar]
- 5.Bolton, H., Jr., J. K. Fredrickson, and L. F. Elliott. 1992. Microbial ecology of the rhizosphere, p. 27-63. In F. B. Metting (ed.), Soil microbial ecology: applications in agricultural and environmental management. Marcel Dekker, Inc., New York, NY.
- 6.Bowen, D., P. J. Dart, P. Ryan, and C. E. Harwood. 1998. Rhizobium inoculants for Australian Acacia species, p. 368-370. In J. W. Turnbull, H. R. Crompton, and K. Pinyopusarerk (ed.), Recent developments in acacia planting. Proceedings of the ACIAR International Workshop, Hanoi, Vietnam, 27-30 October 1997, no. 82. Australian Centre for International Agricultural Research, Canberra, Australia.
- 7.Callaway, R. M., and W. M. Ridenour. 2004. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2:436-443. [Google Scholar]
- 8.Carpenter, A. T., and M. F. Allen. 1988. Responses of Hedysarum boreale Nutt. to mycorrhizas and Rhizobium: plant and soil nutrient changes in a disturbed shrub-steppe. New Phytol. 109:125-132. [Google Scholar]
- 9.Colpaert, J. V., and K. K. Van Tichelen. 1996. Decomposition, nitrogen and phosphorus mineralization from beech leaf litter colonized by ectomycorrhizal or litter-decomposing basidiomycetes. New Phytol. 134:123-132. [Google Scholar]
- 10.Cornet, F., H. G. Diem, and Y. R. Dommergues. 1982. Effet de l'inoculation avec Glomus mosseae sur la croissance d'Acacia holosericea en pépinière et après transplantation sur le terrain, p. 287-293. In V. Gianinazzi-Pearson and S. Gianinazzi (ed.), Les mycorhizes: biologie et utilisation. INRA, Dijon, France.
- 11.Cossalter, C. 1986. Introducing Australian acacias in dry tropical Africa, p. 118-122. In J. W. Turnbull (ed.), Australian acacia in developing countries. ACIAR, Canberra, Australia.
- 12.Culhane, A. C., G. Perriere, E. C. Considin, T. G. Cotter, and D. G. Higgins. 2002. Between-group analysis of microarray data. Bioinformatics 18:1600-1608. [DOI] [PubMed] [Google Scholar]
- 13.Danso, S. K. A., G. D. Bowen, and N. Sanginga. 1992. Biological nitrogen fixation in trees in agro-ecosystems. Plant Soil 141:177-196. [Google Scholar]
- 14.Danso, S. K. A., F. Zapata, and K. O. Awonaike. 1995. Measurement of biological N2 fixation in field-grown Robinia pseudoacacia L. Soil Biol. Biochem. 27:415-419. [Google Scholar]
- 15.Degens, B. P., and J. A. Harris. 1997. Development of a physiological approach to measuring the metabolic diversity of soil microbial communities. Soil Biol. Biochem. 29:1309-1320. [Google Scholar]
- 16.Degens, B. P., and M. Vojvodic-Vukovic. 1999. A sampling strategy to assess the effects of land use on microbial functional diversity in soils. Aust. J. Soil Res. 37:593-601. [Google Scholar]
- 17.Degens, B. P., L. A. Schipper, G. P. Sparling, and M. Vojvodic-Vukovic. 2000. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 32:189-196. [Google Scholar]
- 18.Degens, B. P., L. A. Schipper, G. P. Sparling, and L. C. Duncan. 2001. Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol. Biochem. 33:1143-1153. [Google Scholar]
- 19.De La Cruz, R. E., and M. U. Garcia. 1991. Nitrogen fixation and mycorrhizae in acacias on degraded grassland, p. 59-71. In K. Awang and D. A. Taylor (ed.), Tropical acacias in East Asia and the Pacific. Winrock International, Bangkok, Thailand.
- 20.Del Val, C., J. M. Barea, and C. Azcon-Aguilar. 1999. Diversity of arbuscular mycorrhizal fungus populations in heavy-metal-contaminated soils. Appl. Environ. Microbiol. 65:718-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dighton, J., and P. A. Mason. 1985. Mycorrhizal dynamics during forest tree development, p. 117-139. In D. Moore et al. (ed.), Developmental biology of higher fungi. Cambridge University Press, Cambridge, United Kingdom.
- 22.Dolédec, S., and D. Chessel. 1987. Rythmes saisonniers et composantes stationnelles en milieu aquatique I—description d'un plan d'observations complet par projection de variables. Acta Oecol. 8:403-426. [Google Scholar]
- 23.Duponnois, R., and J. Garbaye. 1991. Techniques for controlled synthesis of the Douglas fir-Laccaria laccata ectomycorrhizal symbiosis. Ann. For. Sci. 48:239-251. [Google Scholar]
- 24.Duponnois, R., H. Founoune, D. Lesueur, J. Thioulouse, and M. Neyra. 2000. Ectomycorrhization of six Acacia auriculiformis provenances from Australia, Papua New Guinea and Senegal in glasshouse conditions: effect on the plant growth and on the multiplication of plant parasitic nematodes. Aust. J. Exp. Agric. 40:443-450. [Google Scholar]
- 25.Duponnois, R., C. Plenchette, J. Thioulouse, and P. Cadet. 2001. The mycorrhizal soil infectivity and arbuscular mycorrhizal fungal spore communities in soils of different aged fallows in Senegal. Appl. Soil Ecol. 17:239-251. [Google Scholar]
- 26.Duponnois, R., C. Plenchette, and A. M. Bâ. 2001. Growth stimulation of seventeen fallow leguminous plants inoculated with G. aggregatum in Senegal. Eur. J. Soil Biol. 37:181-186. [Google Scholar]
- 27.Duponnois, R., and C. Plenchette. 2003. A mycorrhiza helper bacterium (MHB) enhances ectomycorrhizal and endomycorrhizal symbiosis of Australian Acacia species. Mycorrhiza 13:85-91. [DOI] [PubMed] [Google Scholar]
- 28.Duponnois, R., S. Diédhiou, J. L. Chotte, and M. O. Sy. 2003. Relative importance of the endomycorrhizal and/or ectomycorrhizal associations in Allocasuarina and Casuarina genera. Can. J. Microbiol. 49:281-287. [DOI] [PubMed] [Google Scholar]
- 29.Duponnois, R., H. Founoune, D. Masse, and R. Pontanier. 2005. Inoculation of Acacia holosericea with ectomycorrhizal fungi in a semiarid site in Senegal: growth response and influences on the mycorrhizal soil infectivity after 2 years plantation. For. Ecol. Manag. 207:351-362. [Google Scholar]
- 30.Duponnois, R., C. Plenchette, Y. Prin, M. Ducousso, M. Kisa, A. M. Bâ, and A. Galiana. 2007. Use of mycorrhizal inoculation to improve reafforestation process with Australian Acacia in Sahelian ecozones. Ecol. Eng. 29:105-112. [Google Scholar]
- 31.Founoune, H., R. Duponnois, A. M. Bâ, and F. El Bouami. 2002. Influence of the dual arbuscular endomycorrhizal/ectomycorrhizal symbiosis on the growth of Acacia holosericea (A. Cunn. Ex G. Don) in glasshouse conditions. Ann. For. Sci. 59:93-98. [Google Scholar]
- 32.Frey-Klett, P., M. Chavatte, M. L. Clausse, S. Courrier, C. Le Roux, J. Raaijmakers, M. G. Martinotti, J. C. Pierrat, and J. Garbaye. 2005. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 165:317-328. [DOI] [PubMed] [Google Scholar]
- 33.Gadgil, R. L., and P. D. Gadgil. 1971. Mycorrhiza and litter decomposition. Nature 233:133. [DOI] [PubMed] [Google Scholar]
- 34.Gadgil, R. L., and P. D. Gadgil. 1975. Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. N. Z. J. For. Sci. 5:33-41. [Google Scholar]
- 35.Garcia, C., A. Roldan, and T. Hernandez. 1997. Changes in microbial activity after abandonment of cultivation in a semiarid Mediterranean environment. J. Environ. Qual. 26:285-291. [Google Scholar]
- 36.Grayston, S., and C. Campbell. 1996. Functional biodiversity of microbial communities in the rhizosphere of hybrid larch Larix eurolepis and Sitka spruce Picea sitchensis. Tree Physiol. 16:1031-1038. [DOI] [PubMed] [Google Scholar]
- 37.Grayston, S., G. Griffith, J. Mawdsley, C. Campbell, and R. Bardgett. 2001. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 33:533-551. [Google Scholar]
- 38.Heinemeyer, O., H. Insam, E. A. Kaiser, and G. Walenzik. 1989. Soil microbial biomass and respiration measurements: an automated technique based on infrared gas analysis. Plant Soil 116:77-81. [Google Scholar]
- 39.Jeffries, P., S. Gianinazzi, S. Perotto, K. Turnau, and J. M. Barea. 2003. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 37:1-16. [Google Scholar]
- 40.Kisa, M., A. Sanon, J. Thioulouse, K. Assigbetse, S. Sylla, R. Spichiger, L. Dieng, J. Berthelin, Y. Prin, A. Galiana, M. Lepage, and R. Duponnois. 2007. Arbuscular mycorrhizal symbiosis can counterbalance the negative influence of the exotic tree species Eucalyptus camaldulensis on the structure and functioning of soil microbial communities in a sahelian soil. FEMS Microbiol. Ecol. 62:32-44. [DOI] [PubMed] [Google Scholar]
- 41.Kourtev, P. S., J. G. Ehrenfeld, and W. Huang. 2002. Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152-3166. [Google Scholar]
- 42.Kourtev, P. S., J. G. Ehrenfeld, and M. Haggblom. 2003. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biol. Biochem. 35:895-905. [Google Scholar]
- 43.Krebs, C. J. 1989. Ecology methodology. Harper Collins Publishers, New York, NY.
- 44.Lal, R. 1996. Deforestation and land-use effects on soil degradation and rehabilitation in western Nigeria. III. Runoff, soil erosion and nutrient loss. Land Degrad. Dev. 7:99-119. [Google Scholar]
- 45.Lebreton, J. D., R. Sabatier, G. Banco, and A. M. Bacou. 1991. Principal component and correspondence analyses with respect to instrumental variables: an overview of their role in studies of structure-activity and species-environment relationships, p. 85-114. In J. Devillers and W. Karcher (ed.), Applied multivariate analysis in SAR and environmental studies. Kluwer Academic Publishers, Norwell, MA.
- 46.Linderman, R. G. 1988. Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology 78:366-371. [Google Scholar]
- 47.Magurran, A. E. 1988. Ecological diversity and its measurement. Croom Helm, London, United Kingdom.
- 48.Marx, D. H. 1969. The influence of ectotropic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153-163. [PubMed] [Google Scholar]
- 49.Masse, D., P. Cadet, J. L. Chotte, M. Diatta, C. Floret, N. N′Diaye-Faye, E. Pate, R. Pontanier, J. Thioulouse, and C. Villenave. 1998. Jachères naturelles et restauration des propriétés des sols en zone semi-aride. Cas du Sénégal. Agric. Dev. 18:31-38. [Google Scholar]
- 50.Olsen, S. R., C. V. Cole, F. S. Watanabe, and L. A. Dean. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate, p. 19. U.S. Department of Agriculture circular, vol. 939. U.S. Department of Agriculture, Washington, DC. [Google Scholar]
- 51.Olsson, P. A., M. Chalot, E. Baath, R. D. Finlay, and B. Soderstrom. 1996. Ectomycorrhizal mycelia reduce bacterial activity in a sandy soil. FEMS Microbiol. Ecol. 21:77-86. [Google Scholar]
- 52.Parrotta, J. A., J. W. Turnbull, and N. Jones. 1997. Catalyzing native forest regeneration on degraded tropical lands. For. Ecol. Manag. 99:1-7. [Google Scholar]
- 53.Pedley, L. 1986. Australian acacia: taxonomy and phytogeography, p. 11-16. In J. W. Turnbull (ed.), Australian acacia in developing countries. ACIAR, Canberra, Australia.
- 54.Rao, C. R. 1964. The use and interpretation of principal component analysis in applied research. Sankhya 26:329-359. [Google Scholar]
- 55.Reddell, P., and R. Warren. 1986. Inoculation of acacia with mycorrhizal fungi: potential benefits, p. 50-53. In J. W. Turnbull (ed.), Australian acacia in developing countries. ACIAR, Canberra, Australia.
- 56.Richardson, D. M., N. Allsopp, C. M. D'Antonio, S. J. Milton, and M. Rejmanek. 2000. Plant invasion—the role of mutualisms. Biol. Rev. Camb. Philos. Soc. 75:65-93. [DOI] [PubMed] [Google Scholar]
- 57.Rinaudo, T., M. Burt, and C. Harwood. 1995. Growth and seed production of Australian Acacia species at Maradi, Niger. ACIAR For. Newsl. 19:1-2. [Google Scholar]
- 58.Schnürer, T., and T. Rosswall. 1982. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 43:1256-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sieverding, E. 1991. Vesicular-arbuscular mycorrhiza management in tropical agrosystems, p. 371. GTZ, Eschborn, Germany.
- 60.Sparling, G. P. 1995. The substrate induced respiration method, p. 397-404. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, London, United Kingdom.
- 61.Stevenson, B. A., G. P. Sparling, L. A. Schipper, B. P. Degens, and L. C. Duncan. 2004. Pasture and forest soil microbial communities show distinct patterns in their catabolic respiration responses at a landscape scale. Soil Biol. Biochem. 36:49-55. [Google Scholar]
- 62.Thioulouse, J., D. Chessel, S. Dolédec, and J. M. Olivier. 1997. ADE-4: a multivariate analysis and graphical display software. Stat. Comput. 7:75-83. [Google Scholar]
- 63.van der Heijden, M. G. A., and J. H. C. Cornelissen. 2002. The critical role of plant-microbe interactions on biodiversity and ecosystem functioning: arbuscular mycorrhizal associations as an example, p. 181-194. In M. Loreau, S. Naeem, and P. Inchausti, (ed.), Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, London, United Kingdom.
- 64.van der Heijden, M. G. A., R. Bakker, J. Verwaal, T. R. Scheublin, M. Rutten, R. van Logtestijn, and C. Staehelin. 2006. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol. Ecol. 56:178-187. [DOI] [PubMed] [Google Scholar]
- 65.van der Heijden, M. G. A., J. N. Klironomos, M. Ursic, P. Poutoglis, R. Streitwolf-Engel, T. Boller, A. Wiemken, and I. R. Sanders. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69-72. [Google Scholar]
- 66.van der Heijden, M. G. A., T. Boller, A. Wiemken, and I. R. Sanders. 1998b. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082-2091. [Google Scholar]
- 67.Walker, C. 1983. Taxonomic concepts in the Endogonaceae. I. Spore wall characteristics in species description. Mycotaxon 18:443-455. [Google Scholar]
- 68.West, A. W., and G. P. Sparling. 1986. Modifications to the substrate-induced respiration method to permit measurements of microbial biomass in soils of different water contents. J. Microbiol. Methods 5:177-189. [Google Scholar]
- 69.Zhu, W., and J. G. Ehrenfeld. 1996. The effects of mycorrhizal roots on litter decomposition, soil biota, and nutrients in a spodosolic soil. Plant Soil 179:109-118. [Google Scholar]