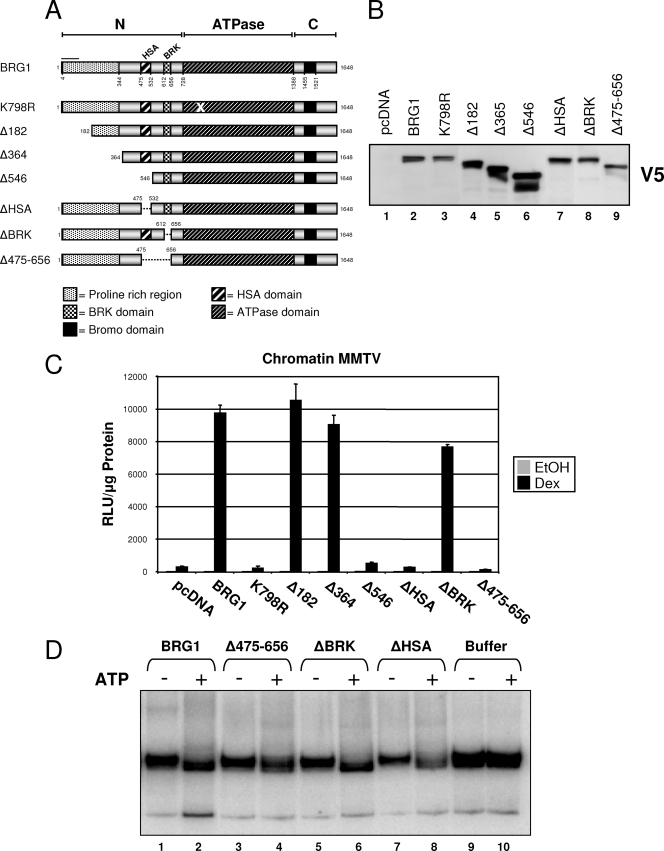
FIG. 2.
Characterization of BRG1 N-terminal truncation/deletion mutants. (A) Schematic representation of BRG1 N-terminal mutants. BRG1 mutant expression plasmids were constructed by truncating amino acids or selectively deleting previously identified domains within the amino-terminal (N-terminal) region of BRG1. Each protein contains a C-terminal V5 epitope tag. (B) Protein expression of BRG1 N-terminal mutants in transfected SW-13 cells using nuclear lysate and anti-V5 antibody. (C) SW-13/MMTV cells were transfected with expression plasmids for GR and pcDNA (empty vector), BRG1, or mutant proteins, treated with Dex, and assayed for luciferase activity. Relative luciferase expression values were normalized to total protein measured (measured in relative light units [RLU] per microgram of protein). Data represented are from three independent experiments. EtOH, ethanol. (D) In vitro remodeling activity of BRG1 N-terminal mutant proteins. Nucleosome mobility assay was performed. BRG1 mutant proteins were purified from 293T cell nuclear extracts, immobilized on V5 beads, and assayed for remodeling activity using a 202-bp DNA fragment, assembled in mononucleosomes with recombinant human histones. Remodeled products were resolved on 5% native polyacrylamide gels.