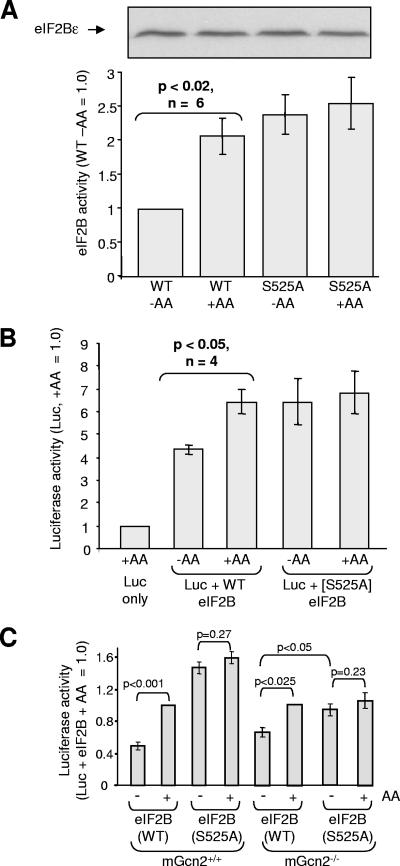
FIG. 7.
Ser525 in eIF2Bɛ plays a key role in regulating eIF2B and protein synthesis. (A) HEK293 cells were transfected with vectors for all five subunits of eIF2B, including either wild-type eIF2Bɛ or the S525A mutant. Cells were subsequently transferred to D-PBS with or without amino acids (AA). Two hours later, cells were lysed, eIF2B complexes were isolated and their activities were determined (n = 6). The panel above the graph shows an immunoblot for myc-eIF2Bɛ. (B) As in in panel A, but cells were cotransfected with the vector for destabilized luciferase. The luciferase activity was measured (n = 4). A blot for myc-tagged eIF2Bɛ is shown above panel A. Other eIF2B subunits were also expressed at similar levels in all four samples (not shown). (C) MEFs (mGcn+/+ or mGcn2−/−, as indicated) were transfected with eIF2B and luciferase vectors as in panel B, but using GenePORTER (Genlantis, San Diego, CA). Cells were subsequently transferred to D-PBS with or without amino acids (AA) for 90 min (the time at which intracellular amino acid depletion is maximal) (3, 38). The luciferase activity was measured (n = 5 to 6). Again, immunoblot analysis revealed that wild-type and S525A eIF2Bɛ were expressed at similar levels (data not shown).