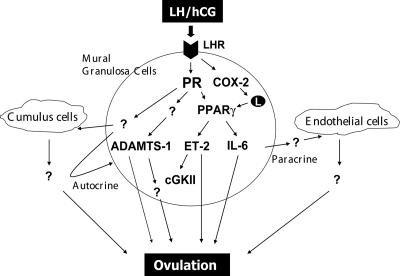
FIG. 10.
A network of PR- and PPARγ-regulated genes controls ovulation. Ovulatory action is initiated by LH, which acts via its receptor on mural granulosa cells of the preovulatory follicles to induce expression of PR in these cells. PR, in turn, induces the expression of PPARγ, a ligand-inducible transcription factor, which is activated by fatty acid metabolites generated by LH-induced COX-2. Activated PPARγ mediates PR function by controlling the expression of a subset of PR target genes, including ET-2, cGKII, and IL-6. It is likely that additional PR-dependent pathways, which are not regulated by PPARγ, such as ADAMTS-1, also contribute to the process of follicular rupture. The products of certain of the PPARγ target genes, such as ET-2 and IL-6, are thought to be released from mural granulosa cells of the preovulatory follicles and act on other ovarian cell types, such as endothelial cells of blood vessels and cumulus cells, via a paracrine mode. They may also act on mural granulosa cells via autocrine mechanisms. We envision that the concerted action of these autocrine and paracrine effectors ultimately brings about ovulation.