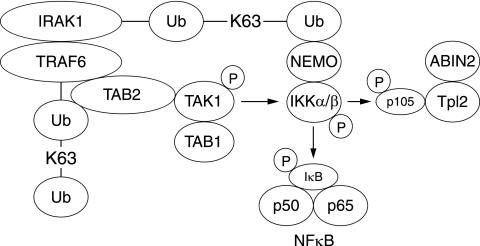
FIG. 7.
Suggested model for how TAK1 and IKKα/β colocalize with the TRAF6-IRAK1 complex. The results presented in this paper, together with data from other laboratories, suggest that IL-1 induces the binding of the TAB2-TAK1-TAB1 complex to K63-pUb-TRAF6, while NEMO-IKK binds to K63-pUb-IRAK1 in the TRAF6-IRAK1 complex. These interactions may colocalize TAK1 and IKKα/β to this complex, which may facilitate the phosphorylation of IKKα/β by TAK1. Once activated, IKK phosphorylates the IκBα component of the NF-κB complex and the p105 regulatory subunit of Tpl2. This triggers the proteasomal destruction of these proteins, leading to the activation of NF-κB and Tpl2.