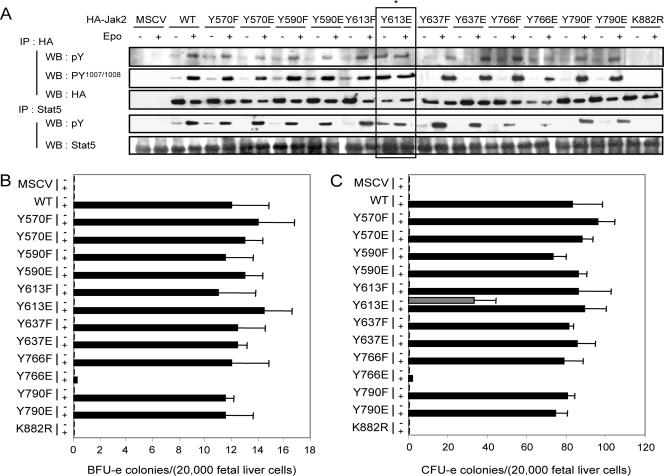
FIG. 2.
Biological activity associated with various tyrosine-to-phenylalanine or -glutamic acid mutants of the JH2 domain of Jak2. (A) Biological activity associated with mutants of Jak2 in which the various tyrosines have been mutated for either a phenylalanine or a glutamic acid. Jak2-deficient mouse embryonic fibroblasts were infected with retroviruses encoding EpoR together with retroviruses encoding HA-tagged Jak2 (WT), the indicated mutants of Jak2, or retroviruses not encoding Jak2 (murine stem cell virus [MSCV]). Forty-eight hours after infection, cells were serum deprived for 16 to 24 h and then stimulated or not with Epo (5 U/ml) for 15 min before solubilization. Cell lysates were subjected to immunoprecipitation using an anti-HA or anti-STAT5 antibody. Immunoprecipitated proteins were then analyzed by Western blotting using antibodies directed against phosphorylated tyrosines (4G10 and pY), activating tyrosine phosphorylation of Jak2 (pY1007/Y1008), the HA epitope on Jak2 (HA), and STAT5. Note the constitutive global tyrosine phosphorylation of Jak2, activating tyrosine phosphorylation of Jak2, and the phosphorylation of STAT5 in cells expressing EpoR together with Jak2-Y613E in the absence of Epo stimulation (*). (B and C) Epo-independent formation of CFU-e from Jak2-deficient fetal liver cells expressing Jak2-V613E. Jak2-deficient fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the absence or presence of Epo (0.2 U/ml) (B) or Epo (3 U/ml) and IL-3 (10 ng/ml) (C). The benzidine-positive CFU-e and BFU-e colonies were scored at day 3 and day 8, respectively.