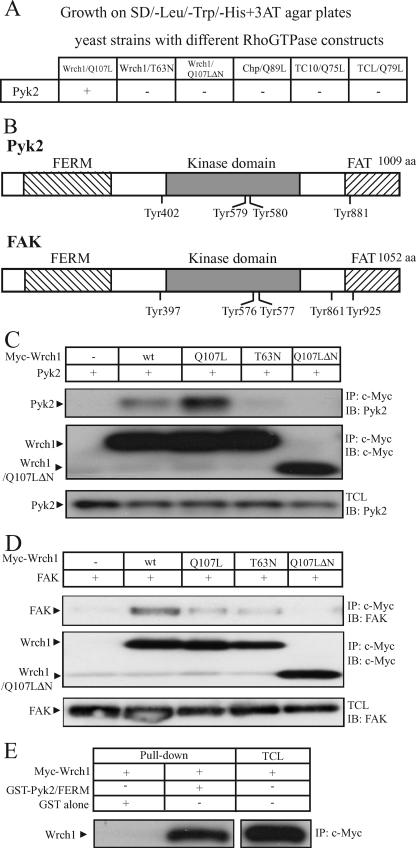
FIG. 2.
Wrch1 binds the Pyk2 and FAK nonreceptor tyrosine kinases. (A) Yeast two-hybrid assay for the interaction of the Cdc42 subfamily members with Pyk2. Interaction of GAL4 activation domain fusion proteins of Pyk2 with GAL4DB-Wrch1(Q107L), GAL4DB-Wrch1(T63N), GAL4DB-Wrch1(Q107LΔN), GAL4DB-ChpL89, GAL4DB-TC10L75, or GAL4DB-TCLL79 in the yeast two-hybrid system. Colonies growing on selective media as described in Materials and Methods were assayed for β-galactosidase activity. A positive interaction is scored as +. (B) Schematic representation of Pyk2 and FAK. aa, amino acids. (C) Interaction between Myc-tagged Wrch1 or Wrch1 mutants and Pyk2 was assessed by transient transfection into HEK293T cells. The presence of Pyk2 in Myc immunoprecipitates (IP) was determined by immunoblotting (IB). wt, wild type. (D) Interaction between Myc-tagged Wrch1 or Wrch1 mutants and FAK was assessed by transient transfections in HEK293T cells. The presence of FAK in Myc immunoprecipitates was determined by immunoblotting. (E) Interaction between the FERM domains of Pyk2 and Wrch1 was determined by incubating lysates from HEK293T cells transiently transfected with Myc-Wrch1 with Pyk2 fusion-proteins bound to glutathione-Sepharose beads (GST alone and GST-FERM). The presence of Myc-Wrch1 in the precipitate was detected by Western blotting employing a Flag-specific antibody.