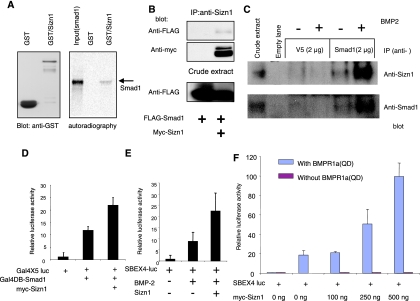
FIG. 2.
Sizn1 up-regulates BMP signaling through interaction with Smad1. (A) GST pull-down assay shows that the GST-Sizn1 fusion protein purified from E. coli binds with in vitro-translated Smad1 protein (a reverse GST pull-down assay using GST-Smad1 confirms this interaction [see the supplemental material]). (B) Immunoprecipitation (IP) and immunoblot analysis of HEK293T cells, transfected with pMIWIII/Sizn1-myc and pCMV/Flag-Smad1, indicate that Sizn1 can interact with Smad1. Sizn1 protein expressed in HEK293T cells showed two bands on the Western blot, the upper band reflecting a phosphorylated form (data not shown). (C) Endogenous Sizn1 interacts with Smad1 that has translocated to the nucleus. A nuclear fraction of BMP-2 (20 ng/ml for 40 min)-treated SN56 cells, which express both Sizn1 and Smad1, was immunoprecipitated with anti-Smad1 antibody (A-4), followed by Western blotting with anti-Sizn1 or anti-Smad1 antibody. The first lane shows that Sizn1 and Smad1 are present in whole lysates. The remaining lanes are immunoblots of nuclear extracts. Anti-V5 antibody was used as a negative control for immunoprecipitation. (D) The Gal4x5-luc reporter assay shows that Sizn1 enhances Smad1 function in the nucleus and that Sizn1 interacts with Smad1 in the nucleus (n = 8). (E) Sizn1 can activate the BMP-2-responsive induction of SBEx4-luc. C2C12 cells were transfected with SBEx4-luc and mock DNA or myc-Sizn1 in the presence or absence of BMP-2 (10 ng/ml) (n = 6). (F) The Sizn1-mediated activation of BMP signaling is dose dependent. C2C12 cells were cotransfected with and without SBEx4-luc, a constitutively active form of BMPR1a(QD), and with various concentrations of myc-Sizn1 (n = 3) [data not shown for a constitutively active mutant such as BMPR1b(QD) (n = 2)].