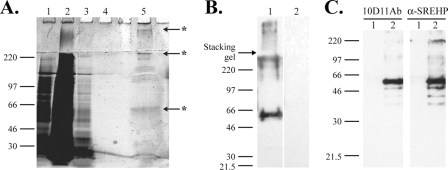
FIG. 6.
Identification of antigen recognized by 10D11Ab. (A and B) Partial purification of the 10D11-reactive antigen by immunoaffinity chromatography. (A) Silver-stained 8% SDS-PAGE gel of proteins applied to and eluted from a 10D11 antibody column. The contents of the numbered lanes are as follows: 1, cleared lysate applied to column; 2, column flowthrough; 3, proteins eluted with first wash; 4, proteins eluted with final wash; 5, eluted 10D11-reactive antigen. Asterisks indicate bands excised and identified by MS. (B) 10D11 antibody immunoblot performed on purified antigen separated by 8% SDS-PAGE. A predominant band of approximately 50 kDa is bound. Lane 1, purified antigen probed with 10D11 antibody; lane 2, purified antigen probed with secondary antibody alone. (C) Specific recognition of the SREHP by 10D11 antibody. Six-His-tagged SREHP or human E3 kinase (negative control) was expressed in E. coli, and bacterial proteins were separated by 10% SDS-PAGE, transferred to a PVDF membrane, and blotted with 10D11 antibody (left) or anti-SREHP ascites (right). Lane 1, His-E3 kinase; lane 2, His-SREHP.