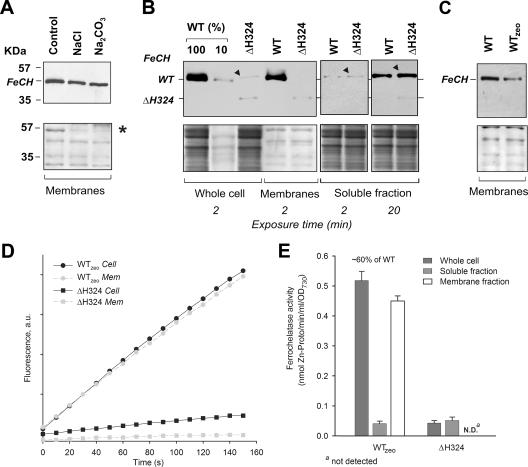
FIG. 3.
Localization, accumulation, and in vitro activity of FeCH in Synechocystis strains. (A) Membranes prepared from the WT strain were washed with 2 M NaCl, pH 11, or 0.1 M Na2CO3 and centrifuged, and the pellet was separated by SDS-PAGE, blotted, and cross-reacted with anti-FeCH antibody. The lower panel shows the membrane stained with Ponceau red as a loading control; the peripheral subunit of ATPase, included as a marker protein for removal by salt washing, is indicated by an asterisk. (B) Whole-cell extract, membrane protein fraction, and soluble fraction from analyzed strains were prepared as described in Materials and Methods. The amount of protein loaded for each sample corresponded to 150 μl of cells at an OD730 of 1. FeCH was detected by an anti-FeCH antibody; the exposure time is indicated. The band resulting from an unspecific cross-reaction is marked by a black triangle. Below is the membrane stained with Ponceau red. (C) Different amounts of the FeCH in membrane fractions of the WT and WTzeo strains. Samples were prepared, and the FeCH was detected as described above. (D) In vitro FeCH activity in whole-cell extract fractions (Cell; black symbols) and in membranes (Mem; gray symbols) as determined by continuous spectrofluorometric assay. Activities were monitored as an increase in fluorescence of zinc-Proto using excitation and emission wavelengths of 420 and 590 nm, respectively. (E) Comparison between FeCH activities measured in whole-cell extract, the soluble fraction, and the membrane fraction of studied strains. The values are the means of three independent experiments. au, arbitrary units.