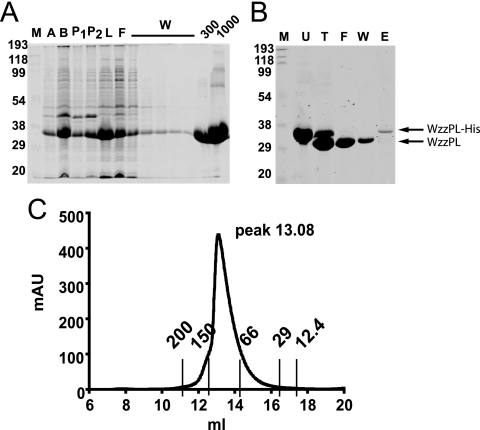
FIG. 1.
Purification of WzzPL6×His. (A) BL21(DE3)/pCM244 cells were induced with 0.75 mM IPTG, WzzPL6×His was purified using Ni2+-Sepharose beads, and the protein was eluted from the column with imidazole. Aliquots were removed at each step of the purification procedure and separated on a 14% SDS-PAGE gel stained with PageBlue (Fermentas). Lane M, broad-range prestained SDS-PAGE standard (Bio-Rad); lane A, cells boiled after induction; lane B, lysate obtained by French press treatment; lane P1, pellet after the first centrifugation at low speed; lane P2, pellet after the second centrifugation at high speed; lane L, supernatant after centrifugation; lane F, flowthrough; lanes W, washes with 50 mM Na2PO4 (pH 7), 300 mM NaCl, 20 mM imidazole; lane 300, elution with 50 mM Na2PO4 (pH 7), 300 mM NaCl, 300 mM imidazole; lane 1000, elution with 50 mM Na2PO4 (pH 7), 300 mM NaCl, 1,000 mM imidazole. (B) Removal of six-His tag from purified WzzPL6×His. Purified WzzPL6×His was incubated overnight with 3 U thrombin per mg of protein. Aliquots were removed at each step of the purification procedure and separated on a 14% SDS-PAGE gel, and the gels were stained with PageBlue (Fermentas). Lane M, broad-range prestained SDS-PAGE standard (Bio-Rad); lane U, untreated; lane T, treated with thrombin; lane F, flowthrough; lane W, washes with 50 mM Na2PO4 (pH 7), 300 mM NaCl, 20 mM imidazole; lane E, elution with 50 mM Na2PO4 (pH 7), 300 mM NaCl, 1,000 mM imidazole. (C) Size exclusion chromatography profile. Three milligrams of purified WzzPL protein was applied to a Superdex 200 10/300 GL column equilibrated with 50 mM Na2PO4 (pH 7), 100 mM NaCl. The column was calibrated with the following MWGF-200 (Sigma) molecular mass markers: cytochrome c (12.4 kDa; 17.29 ml), carbonic anhydrase (29 kDa; 16.05 ml), albumin (66 kDa; 14.02 ml), alcohol dehydrogenase (150 kDa; 12.47 ml), and β-amylase (200 kDa; 12.5 ml). The height of the peak depended on the relative concentration of the sample loaded into the column. The elution peak (13.08 ml) corresponds to a molecular mass of approximately 100 kDa. The y axis indicates the absorption at 280 mm (in milliabsorbance units [mAU]) as determined with the detector. The x axis indicates the volume (in milliliters) passed over the column.