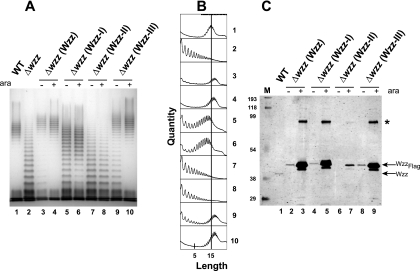
FIG. 4.
(A) LPS production in E. coli K-12 strain W3110 (WT) and isogenic mutant EVV33 (Δwzz) complemented with plasmids expressing wild-type Wzz (Wzz) and forms with mutations in each of the coiled-coil regions (Wzz-I, Wzz-II, and Wzz-III). LPS was prepared from cultures not induced and induced with 0.2% (wt/vol) arabinose (ara). Samples were separated on a 12.5% Tris-glycine SDS-PAGE gel, and the gel was stained with silver. Lane 1, W3110(pMF19); lane 2, EVV33(pMF19); lanes 3 and 4, EVV33(pMF19/pEH1); lanes 5 and 6, EVV33(pMF19/pEH6); lanes 7 and 8, EVV33(pMF19/pEH4); lanes 9 and 10, EVV33(pMF19/pEH5). (B) Densitometric analysis of samples shown in panel A. The analysis was performed with the program ImageJ as described in Materials and Methods. The vertical line in all graphs indicates the mean chain length of wild-type strain W3110(pMF19). The numbers on the right correspond to the lane numbers in panel A. (C) Wzz expression in cells cultured under the conditions used for the LPS extracts (see Materials and Methods). Total membranes were separated on a 14% SDS-PAGE gel. Proteins were transferred to a nitrocellulose membrane, and the blot was reacted with affinity-purified rabbit polyclonal anti-WzzPL and anti-Flag antisera. WzzPL-specific bands were detected by fluorescence using IRDye800CW anti-rabbit IgG antibodies, and Flag-specific bands were detected with Alexa Fluor 680 anti-mouse IgG antibodies. Lane M, broad-range prestained SDS-PAGE standard (Bio-Rad); lane 1, W3110(pMF19/pBADFlag); lanes 2 and 3, EVV33(pMF19/pEH1); lanes 4 and 5, EVV33(pMF19/pEH6); lanes 6 and 7, EVV33(pMF19/pEH4); lanes 8 and 9, EVV33 (pMF19/pEH5). The arrows indicate the migration positions of plasmid-encoded WzzFlag and chromosomally encoded Wzz. The asterisk indicates the migration position of a Wzz dimer.