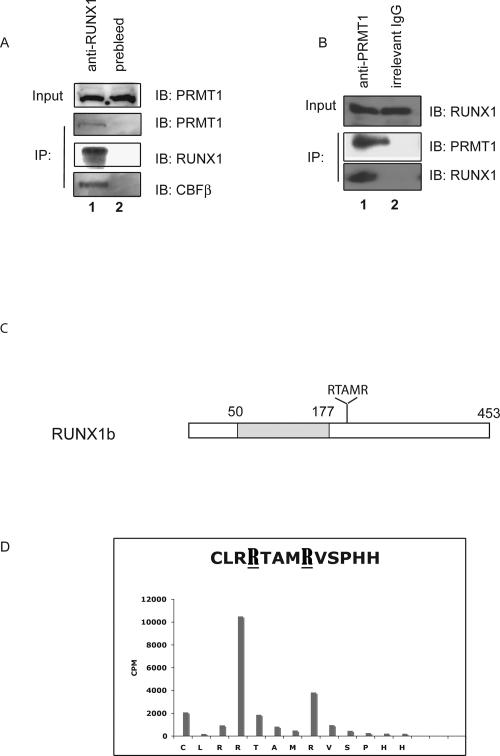
Figure 1.
The endogenous RUNX1 and PRMT1 proteins physically interact in HEL cells. (A) An anti-RUNX1 antibody was used to immunoprecipitate PRMT1 from HEL cell extracts, using preimmune rabbit serum as control. As a positive control, CBFβ was detected among the anti-RUNX1 immunoprecipitated proteins. (B) An anti-PRMT1 murine antibody was used to immunoprecipitate RUNX1 from HEL cell extracts, using an irrelevant murine IgG antibody as negative control. (C) Schematic diagram of RUNX1b, showing the position of the Runt domain shaded and the RTAMR methylation site; the numbers on top correspond to the amino acid position. (D) Results of Edman degradation of an in vitro methylated synthetic peptide that contains amino acids 203–215 of RUNX1. Two sites in RUNX1 that are arginine-methylated by recombinant PRMT1 are identified.