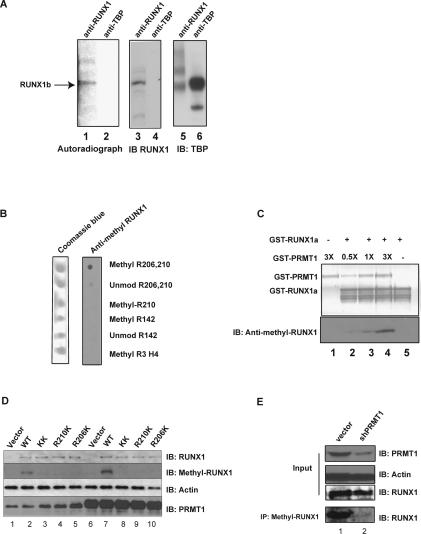
Figure 2.
RUNX1 is arginine-methylated in vivo. (A) HEL cells were treated with protein synthesis inhibitors before [3H-methyl]-methionine was added to the medium for labeling. The cell lysate was then immunoprecipitated with an anti-RUNX1 antibody (lanes 1,3) or an irrelevant (anti-TBP) antiserum (lanes 2,4). Methylated RUNX1 is shown in the left panel, and the middle and right panels are immunoprecipitation controls for the two antibodies used. (B) The anti-methyl-arginine-specific RUNX1 antibody efficiently recognizes the R206 and R210 diasymmetrically methylated peptide, but not the unmethylated peptide, the R210-only asymmetrically methylated peptide, the R142 asymmetrically methylated peptide, or the R3 asymmetrically methylated H4 histone tail peptide in a dot blot analysis. (C) The anti-methyl-arginine RUNX1 antibody recognizes recombinant RUNX1 only after in vitro methylation by recombinant GST-PRMT1. The top panel is the PVDF membrane stained for protein, and the bottom panel is a Western blot assay performed with the anti-methyl-arginine-RUNX1 antibody. (D) The wild-type RUNX1 protein, but not the R206K and R210K mutant proteins, is doubly arginine-methylated in HeLa cells, as detected using the anti-methyl-arginine RUNX1 antibody. Lane 1 is HeLa cells transfected with the empty pCDNA3 vector, whereas lanes 2–5 contain overexpressed wild-type RUNX1c or the various R-to-K RUNX1 mutants. Lane 6 contains HeLa cells that overexpress PRMT1 alone, and lanes 7–10 contain overexpressed RUNX1c and overexpressed PRMT1. The overexpression of PRMT1 leads to greater methylation of RUNX1 wild-type protein (cf. lanes 2 and 7), without changing the level of RUNX1 expression. Similarly, overexpression of RUNX1c did not change the level of PRMT1 (cf. lanes 1 and 2). (E) Knocking down PRMT1 in HEL cells with shRNA reduces the amount of arginine-methylated RUNX1 but not the total amount of RUNX1 protein. Lane 1 is the vector-integrated HEL cells. Lane 2 shows the decrease in PRMT1 and methyl RUNX1 in HEL cells that stably express PRMT1 shRNA. Actin levels serve as the loading control.