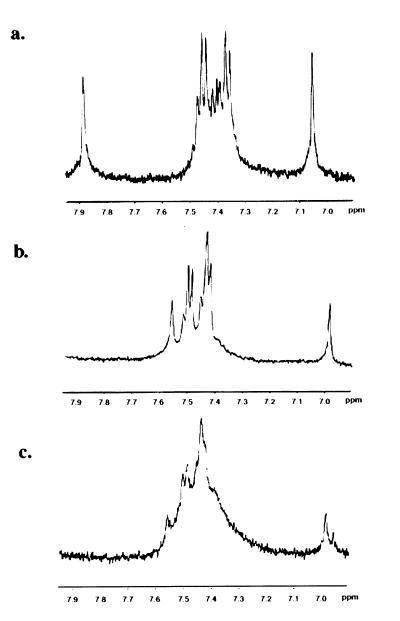
Figure 3.
Cobalt(III) complex disrupts the structure of the zinc finger peptide. 1H NMR data were acquired on a Bruker AM-500 (T = 30°C, 5 mM D2O, pH 7.0); 3.5 mg of peptide were dissolved in 350 μl D2O (Cambridge Isotope Laboratories), for each of the samples. Approximately 1 M equivalent of ZnCl2 was added to the samples in a and b; 1 M equivalent of COBA also was added to c. In the absence of zinc as shown in a, histidine peaks appear at 7.08 and 7.91, which shift upon addition of zinc to those characteristic of metal bound histidine at 7.01 and 7.58 (b). Upon the addition of COBA, the spectrum degrades to a broad peak (c), suggesting a strong perturbation to the structure of the peptide.