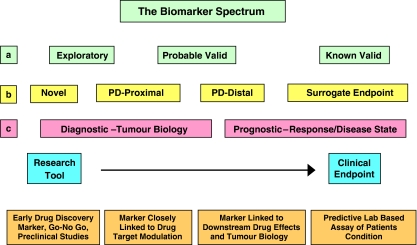
Figure 1.
The Biomarkers spectrum from the perspective of different stakeholders. (a) The US Food and Drug Administration envisages three evidentiary stages towards biomarker approval (Goodsaid and Frueh, 2007). (b) Within the pharmaceutical industry biomarkers are utilized throughout the whole drug discovery process from compound selection to clinical trials (Lee et al., 2005). (c) In cancer medicine, biomarkers may be seen as being either diagnostic of tumour biology, or prognostic of disease or therapeutic outcome (Ludwig and Weinstein, 2005).