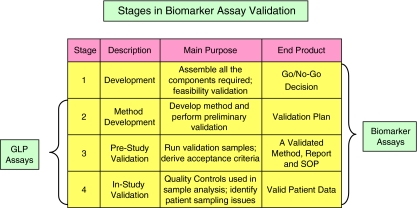
Figure 2.
The stages of biomarker assay validation. Three stages are proposed for bioanalytical techniques referred to as Good Laboratory Practice (GLP) assays, each with a predefined purpose and goal. Biomarker assays require an additional phase where feasibility studies are conducted to determine whether or not to proceed with the particular technique.