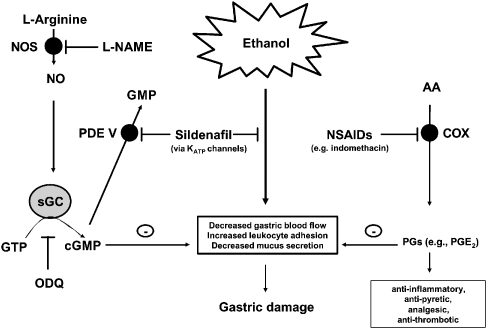
Figure 1.
Ethanol induces gastric mucosal injury through the release of inflammatory mediators which in turn induce vasoconstriction/ischaemia and cell death. Non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin inhibit cyclooxygenase (COX) enzymes to prevent the formation of prostaglandins (PGs) from the membrane lipid arachidonic acid (AA). Products of COX activity, such as PGE2, also act to limit gastric damage by increasing blood flow and reducing leukocyte adhesion. Inhibition of PG formation by NSAIDs therefore results in increased gastropathy. Sildenafil, by inhibiting phosphodiesterase V (PDE V), prevents the breakdown of cGMP to GMP. In addition, it also reduces gastric damage by augmenting gastric blood flow and limiting leukocyte adhesion. Nitric oxide (NO), formed by the action of NOS enzymes on L-arginine, acts upon soluble guanylate cyclase (sGC) to convert GTP to cGMP. Inhibition of sGC by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) reverses any protective effect of sildenafil against ethanol-induced gastric damage. Furthermore, the ATP-sensitive potassium channel (KATP) blocker glibenclamide is capable of reversing sildenafil's gastroprotective effect against ethanol-induced gastric damage suggesting that ATP KATP channels are also involved in regulating gastric protection. Importantly then, sildenafil offers protection against ethanol-induced gastric damage via activation of the NO/cGMP/KATP pathway (Figure adapted from Sawatzky et al., 2005).