Fig. 2.
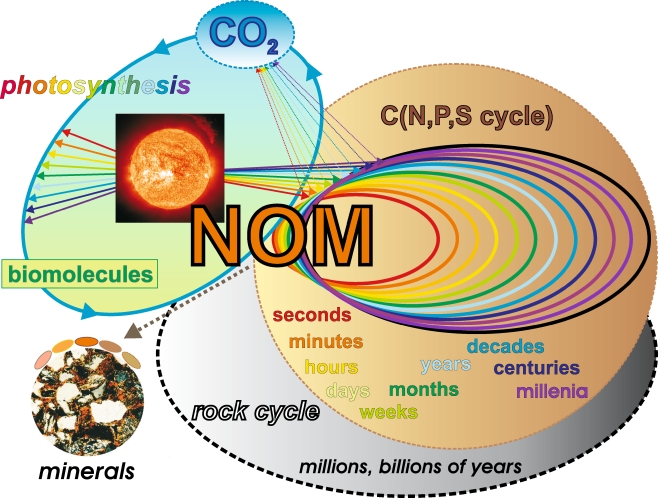
Natural organic matter (NOM) continuously interacts with a broad range of terrestrial, limnic and marine ecosystems. Common to all of these environments are the fundamental molecular aspects of life, and an availability of extended mineral surfaces for interactions with and binding of NOM. The dynamic equilibrium of NOM generation and decomposition spans timescales of many different orders of magnitude (from microseconds to hundreds of thousands of years), and it results from a combined action of biotic and abiotic reactions. NOM may be intrinsically recalcitrant because of the chemical structures of its organic molecules; alternatively, strong NOM–mineral interactions could alter the reactivity of these organic molecules towards increased resistance to degradation. The physical protection of organic matter at interior mineral surfaces provides alternative pathways that enable the recalcitrance of NOM. Photochemical degradation, one of the most significant abiotic reactions of NOM, often results in small molecules like CO2, which are mobile and are easily distributed within various ecosystems. Biomolecules derived from photosynthesis or otherwise originating from a genetic code are eventually decomposed according to the general laws and constraints of thermodynamics and kinetics (Fig. 1). Over very long timescales, the interactions of NOM with minerals at elevated temperatures result in the formation of geopolymers, like kerogen, coal, and oil shales. These ancient materials participate in bio- and geochemical cycling through natural and anthropogenic combustion and through weathering [6]