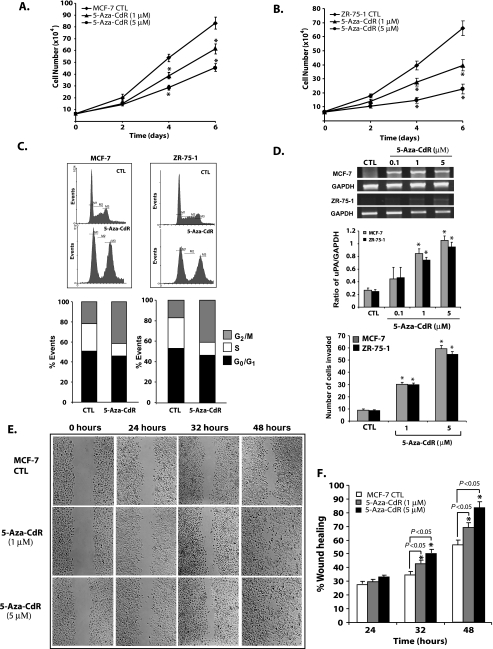
Figure 1.
Effect of 5-aza-CdR treatment on the proliferation, invasion, and migration of noninvasive cell lines. Noninvasive breast cancer cells, MCF-7 and ZR-75-1, were seeded at the same density and subjected to two different doses of 5-aza-CdR or with vehicle control alone (CTL) in a six-well plate, and growth curve analysis was carried out to determine the effect of 5-aza-CdR on the proliferation rate. At each time point, cells were trypsinized and counted as described in the Materials and Methods section (A and B). All data presented are expressed as mean ± SEM of quadruplicate wells for each time point. Similar results were obtained in two independent experiments. Cell cycle distribution analysis was carried out by FACS for control and 5-aza-CdR-treated MCF-7 and ZR-75-1 cells (5 µM for 7 days) after staining the cells with propidium iodide as described in the Materials and Methods section. Data were analyzed by WinMDI version 2.8 and plotted (C). Data are presented as mean of % events from three independent experiments by 100% stacked column graph for control and experimental groups. Expression of uPA, a protease involved in invasion, was analyzed by RT-PCR in MCF-7 and ZR-75-1 cells treated with different doses of 5-aza-CdR (D). Data were presented for control and experimental groups as mean ± SEM values from two independent experiments. The invasive capacity of MCF-7 and ZR-75-1 cells treated with different doses of 5-aza-CdR was determined using a Boyden chamber Matrigel invasion assay. The bar diagram represents the mean ± SEM as described in the Materials and Methods section. Wound healing migration assay was carried out on MCF-7 cells in the absence and presence of 5-aza-CdR (1 and 5µ M) in the culture medium with 2% fetal bovine serum as depicted in panels E and F. Cells were seeded at the same density and grown as monolayer and wounded as discussed in the Materials and Methods section. % Wound healing was recorded at different time points, and percentage of wound healing with respect to T0 was calculated using the described equation. Similar results were obtained from two different experiments. All data were presented as mean ± SEM of duplicate values from control and experimental groups from two independent experiments. Significant difference from the control is represented by an asterisk (*P < .05).