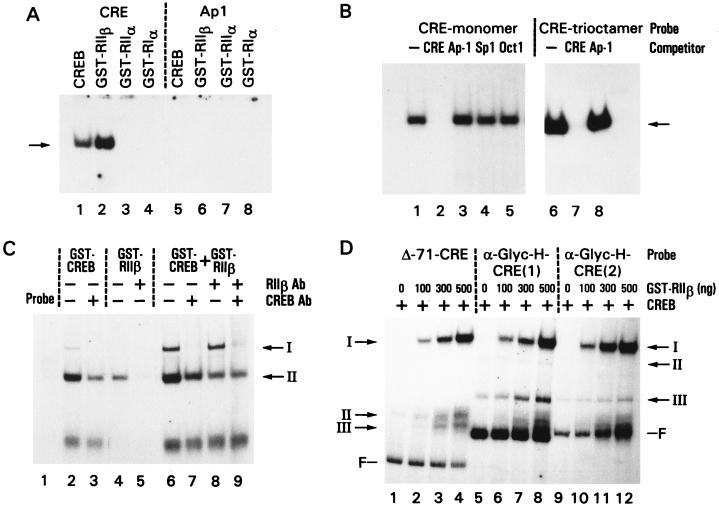
Figure 3.
GST-RIIβ fusion protein binding to the CRE. (A) Gel retardation assay of R subunits (N-terminal parts)-GST proteins. The purified RIIβ-, RIIα-, and RIα-GST proteins were used in the assays. Lanes: 1–4, 32P-labeled CRE probe; 5–8, 32P-labeled AP-1 probe; 1 and 5, 0.5 μg of CREB (CREB-1, bZIP, Santa Cruz Biotechnology); 2 and 6, 3 μg of GST-RIIβ; 3 and 7, 3 μg of GST-RIIα; 4 and 8, 3 μg of GST-RIα. (B) Gel retardation assay of GST-RIIβL (RIIβ whole molecule) protein. Lanes: 1–5, GST-RIIβL (500 ng) CRE-monomer-32P probe in the absence (lane 1) and presence (lanes 2–5) of competitor as indicated; lanes 6–8 contained thrombin-digested GST-RIIβL (500 ng), CRE-trioctamer 32P-probe in the absence (lane 6) and presence (lanes 7 and 8) of competitor as indicated. (C) Gel retardation assay of GST-RIIβL in the presence of GST-CREB. 32P-labeled CRE-monomer was used. Lanes: 2 and 3, GST-CREB (10 ng) in the absence and presence, respectively, of anti-CREB antibody (Santa Cruz Biotechnology); 4 and 5, GST-RIIβL (100 ng) in the absence and presence, respectively, of anti-RIIβ antibody (Transduction Laboratories, Lexington, KY); 6–9, GST-CREB (10 ng) plus GST-RIIβL (100 ng) in the absence and presence of anti-CREB antibody or anti-RIIβ antibody as indicated. (D) Gel retardation assay of GST-RIIβL in the presence of CREB. 32P-labeled probes are as follows. Lanes: 1–4, Δ−71-CRE; 5–8, α-Gly promoter with one CRE [CRE(1)]; 9–12, α-Gly promoter with CRE dimer [CRE(2)]. All lanes contained 10 ng of CREB (CREB-1, bZIP, Santa Cruz Biotechnology) and the indicated amounts of GST-RIIβ protein; lanes 1, 5, and 9 contained CREB only. Bands I-III, GST-RIIβ- or CREB-CRE complexes; F, free probe. The data represent one of three to five experiments that gave similar results.