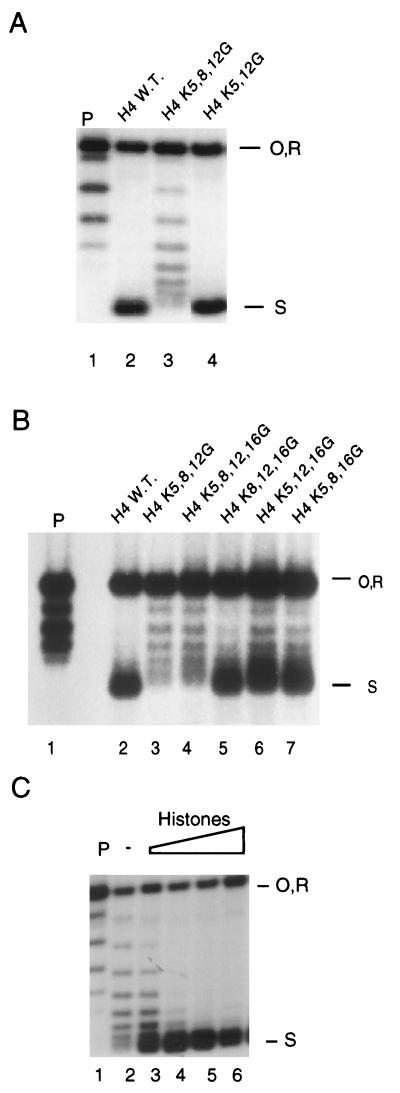
Figure 3.
Nucleosome assembly in vitro. Strains were grown in galactose to logarithmic phase, and then shifted to glucose-containing medium for 12 h to repress expression of wild-type H3 and H4 from plasmid B. Whole cell extracts were prepared from these cells and used for the in vitro nucleosome assembly reaction. The topology of a closed, relaxed, internally labeled plasmid DNA before (P; lane 1, each panel) and after incubation with extracts was analyzed in agarose gels. Plasmid supercoiling was measured after a 2-min assembly reaction. O, R, and S represent open, relaxed, and supercoiled plasmid DNA, respectively. (A) K5/K12 mutagenesis has no evident effect on nucleosome assembly in vitro (compare lane 4 to lane 2). In contrast, K5/K8/K12 mutagenesis severely disrupts nucleosome assembly (lane 3). (B) Redundancy of H4 K5, K8, and K12 in supporting nucleosome assembly in vitro. A comparison of the effects of mutagenesis of K5/K8/K12 (lane 3) and K5/K8/K12/K16 (lane 4) with mutagenesis of K8/K12/K16 (lane 5), K5/K12/K16 (lane 6), and K5/K8/K16 (lane 7). In comparison with wild-type H4 extracts (lane 2), the greatest effects on nucleosome assembly are seen when K5/K8/K12 (or K5/K8/K12/K16) are mutagenized simultaneously (lanes 3 and 4). (C) Extract from a mutant strain lacking H3 and H4 N termini can assemble plasmid when wild-type histones are added back to the assembly-defective extract. Wild-type histones (0, 1, 2, 4, or 6 μg in lanes 2–6) were added to 50 μg of extract. Reactions were performed for 60 min at 30°C.