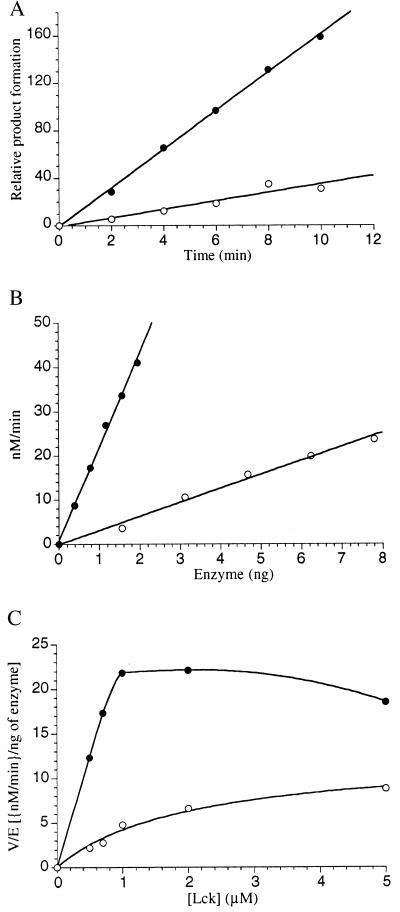
Figure 5.
Phosphorylation of Lck catalyzed by semisynthetic Csk proteins. (A) Time course of CskPEP and CskpPEP-catalyzed phosphorylation of Lck. •, CskpPEP; ○, CskPEP. Relative product formation is corrected for the amount of CskPEP and CskpPEP utilized. [Lck] = 1 μM. (B) Kinase activity vs. CskPEP and CskpPEP concentration with the substrate Lck. •, CskpPEP; ○, CskPEP. [Lck] = 1 μM. Reaction time = 2 min. (C) Rate of phosphorylation of Lck catalyzed by CskPEP and CskpPEP vs. Lck concentration. •, CskpPEP; ○, CskPEP. The data for CskpPEP could not be strictly fit to standard Michaelis-Menten kinetics because of apparent substrate inhibition. For CskpPEP the Km (apparent) (Lck) estimated as the substrate concentration necessary for 50% maximal velocity is 0.4 ± 0.1 μM. The data for CskPEP was fit to the Michaelis-Menten equation and gave Km (apparent) (Lck) = 2 ± 0.5 μM. The Lck Km with wild-type Csk enzyme under these conditions is 3–5 μM (25). All assays were performed as described (25) on a 15 μl scale at 30°C by using catalytically impaired Lck (N-terminal 63 aa deleted, containing a K273R mutation) at fixed and near-saturating ATP concentration [10 μM, ≈3 × Km (apparent)] and optimal MnCl2 concentration (5 mM) under initial conditions (<10% turnover of the limiting substrate). All assays were carried out at least two times and duplicate points generally agreed within 10%. Kinase activity was shown to be identical for the phosphotyrosine-affinity purified and unpurified semisynthetic proteins by using Lck as substrate. Kinase specificity for the 505-tyrosine of Lck was confirmed by demonstrating that 505-phosphorylated Lck was not detectably phosphorylated by either semisynthetic protein using conditions described (25).