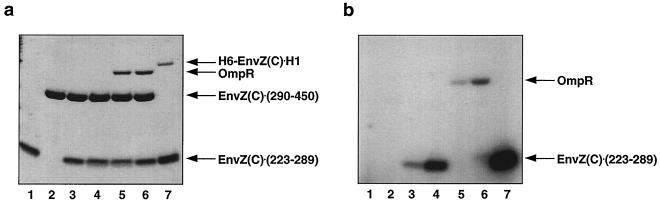
Figure 3.
The recovery of kinase activity by complementation between EnvZ(C)⋅(223–289) and EnvZ(C)⋅(290–450). EnvZ(C)⋅(223–289) (lane 1) or EnvZ(C)⋅(290–450) (lane 2) (1.2 × 10−5 M) was incubated with 0.5 μCi (17 pmol) of [γ-32P]ATP for 20 min. For trans-autophosphorylation of EnvZ(C)⋅(223–289) (1.2 × 10−5 M) by EnvZ(C)⋅(290–450) (1.2 × 10−5 M), two proteins were incubated with 0.5 μCi of [γ-32P]ATP (17 pmol) for 5 min (lane 3) and 20 min (lane 4). For the phosphotransfer reaction to OmpR, EnvZ(C)⋅(223–289) (1.2 × 10−5 M) was first trans-autophosphorylated by EnvZ(C)⋅(290–450) (1.2 × 10−5 M) with 0.5 μCi of [γ-32P]ATP for 5 min (lane 3) and then OmpR (2.4 × 10−6 M) was added into the trans-autophosphorylation mixture, and the mixture was incubated for another 15 s (lane 5) and 15 min (lane 6). Lane 7 shows trans-autophosphorylation of EnvZ(C)⋅(223–289) (1.2 × 10−5 M) by H6-EnvZ(C)⋅H1 (2.4 × 10−6 M) with 0.5 μCi of [γ-32P]ATP for 5 min. All reactions were conducted at room temperature in 20 μl of buffer A and stopped by adding 5× SDS gel loading buffer. Samples were then subjected to SDS/PAGE with a 16% Tricine gel (NOVEX), followed by staining with Coomassie brilliant blue (a) and autoradiography (b).