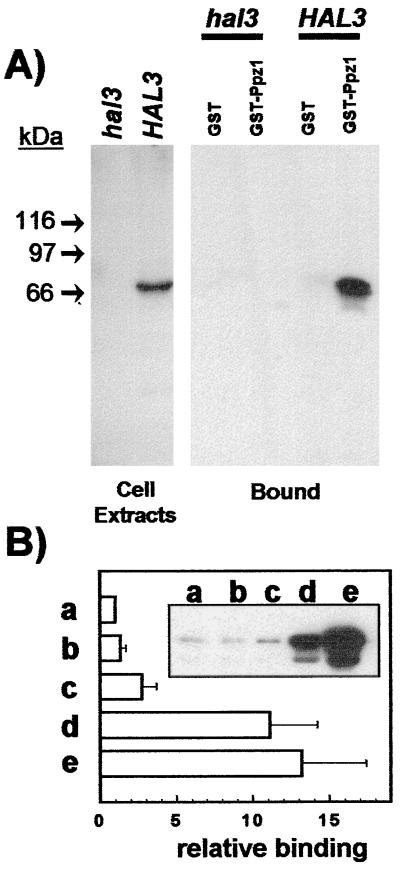
Figure 2.
Physical interaction between bacterially expressed Ppz1p and cellular Hal3p. (A Left) Immunodetection of Hal3p in cell extracts. Whole yeast extracts were prepared as described from strain JA-104 (hal3Δ) or wild-type strain JA-100 (containing the HAL3 gene in a multicopy plasmid). Extracts were subjected to SDS/PAGE and immunoblotted, and the Hal3 protein was detected with polyclonal antibodies. (A Right) Cell extracts (5 mg of total protein) from the above-mentioned strains were mixed with recombinant GST or GST-Ppz1 bound to glutathione-Sepharose beads and processed as described under Materials and Methods. The presence of Hal3p bound to the affinity system was detected by using anti-Hal3p antibodies. (B) Similar binding experiments (using about 1 mg of total yeast proteins) were performed using as a bait: (a) GST-Ppz1, (b) GST-Ppz1(R451L), (c) GST-Ppz1(Δ241–318), (d) GST-Ppz1(Δ17–193), and (e) GST-Ppz1(Δ1–344). Because, in some cases, the preparations of the recombinant proteins contained small quantities of lower-molecular-weight products, the amount of the complete proteins used as a bait was estimated by Coomassie blue staining and/or immunoblot using anti-Ppz1p antibodies. After immunoblot with the anti-Hal3p antibody, the intensity of the signals was corrected, in each case, for possible differences in the amount of the bait proteins. The values correspond to the increase (-fold) with respect to the signal given by the GST-Ppz1 product and are the mean ± SEM of five independent experiments. Inset shows a typical immunoblot experiment.