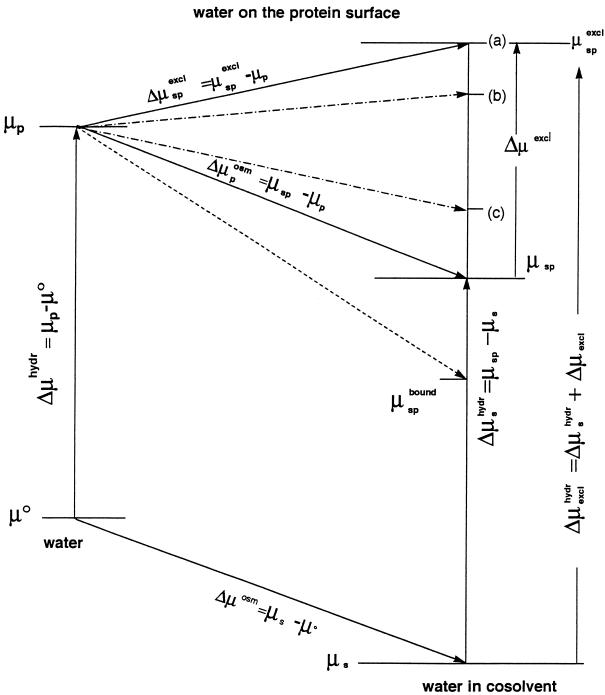
Figure 1.
Thermodynamic diagram of the effects on the standard chemical potential of water of (i) addition of a cosolvent (μs) and (ii) addition of a protein surface element to pure water, μp, and of aqueous solutions of an inert (neutral) cosolvent (μsp) and of an excluded cosolvent (μspexcl). (For comparison, the effect of a bound cosolvent is shown (μspbound) by the short dashed line.) The osmotic effect on water molecules on a protein surface is Δμposm = Δμosm. An excluded cosolvent raises the standard chemical potential by Δμexcl. The dot–dash lines represent cosolvents with different magnitudes of exclusion. The levels (a), (b), and (c) could correspond, for example, to the changes in preferential hydrations measured in the presence of triethylene glycol, betaine, and sucrose during the specific binding of gal repressor (18).