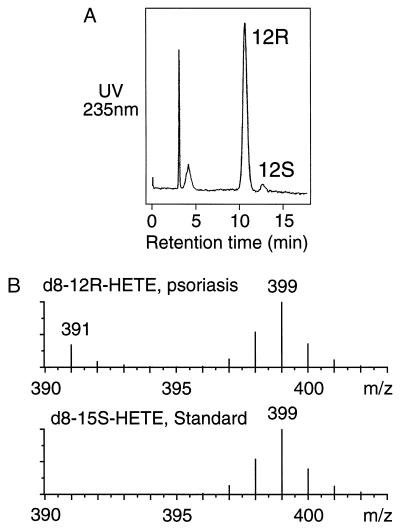
Figure 1.
Analysis of deuterated 12-HETE formed in psoriatic scales. (A) Chiral HPLC of the deuterium-labeled 12-HETE from incubation of deuterated arachidonic acid in psoriatic scales. The 12-HETE was chromatographed as the PFB ester on a Chiralcel OD column using a solvent of hexane/isopropanol (100:5, vol/vol) and detected by UV monitoring at 235 nm. (B) Partial mass spectra of the 12R-HETE from A (Upper), and 15S-HETE prepared from the same batch of deuterated arachidonic acid using the soybean lipoxygenase (Lower). The samples were analyzed by negative ion/chemical ionization GC-MS as the PFB ester trimethylsilyl ether derivatives by repetitive scanning in the range m/z 390–404. The partial mass spectra are the average of all scans collected during elution of the peaks from the GC. Unlabeled 12R-HETE also is detected in the psoriatic sample at m/z 391.