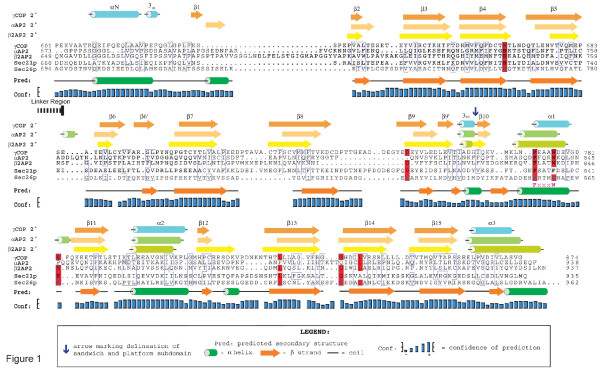
Figure 1.
Alignment of COPI and AP2 appendage domains. A structure-based sequence alignment of the appendage domains of α- and βAP2 with that of γCOP was generated as described in [16]. Secondary structural elements of the γCOP and the AP2 appendage domains determined previously are indicated above the sequence alignment. The blue arrow denotes the position of the boundary between the platform and the Ig-like (β-sandwich) subdomains. Highlighted in red is where four or more sequences contained a strictly conserved residue at that position. Regions of similarity, calculated using functional amino acid groupings, are boxed in blue. Residues for which no structural information is available were positioned using a primary sequence alignment using the ClustalX 1.83 program and manual inspection. The conserved FxxxW motif is indicated below the sequence alignment in pink. The predicted secondary structural elements for the appendage domain of βCOP, Sec26p are shown beneath the alignment as determined with the PSIPRED confidence level of the assignments shown in the diagram.