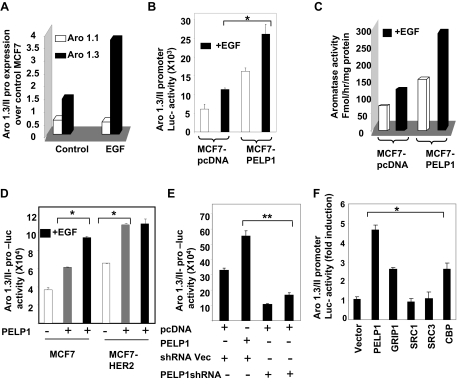
Figure 3.
EGF Enhances PELP1-Mediated Activation of Aromatase
A, MCF7 and MCF7-PELP1 cells were cultured in serum-free medium for 24 h and then treated with or without 100 ng/ml EGF. Total RNA was isolated, and real-time PCR analysis of aromatase gene expression was performed using aromatase exon 1.3- and 1.1-specific primers. B, MCF7 and MCF7-PELP1 cells were transiently transfected with Aro-1.3/II luciferase reporter. After 48 h, cells were cultured in serum-free medium for 24 h, after which they were treated with or without 100 ng EGF/ml for 12 h, and luciferase activity was measured. C, MCF7 and MCF7-PELP1 clone cells were treated with or without EGF, and aromatase activity in control MCF7 and MCF7-PELP1 clones was measured by tritiated-water release assay. D, MCF7 and MCF7-HER2 cells were transiently transfected with Aro-1.3/II reporter gene along with or without PELP1 and treated with or without EGF for 12 h. Luciferase activity was measured. E, MCF7-HER2 cells were cotransfected with Aro-1.3/II reporter along with or without PELP1 shRNA vectors. After 72 h, luciferase reporter activity was measured. F, MCF7 cells were cotransfected with Aro-1.3/II reporter along with PELP1, GRIP1, SRC1, SRC3, and CBP expression vectors. After 72 h, cells were treated with 100 ng/ml EGF for 12 h, and then luciferase reporter activity was measured. *, P < 0.05; **, P < 0.001. Data shown are the means ± se from three independent experiments performed in triplicate wells.