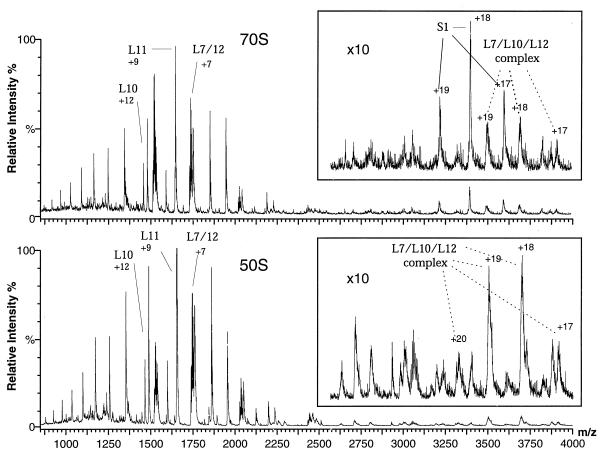
Figure 1.
Positive ion ESI mass spectra of aqueous solutions of intact 70S ribosomes and 50S subunits at pH 6.4. Only the most intense peak for each assigned protein is labeled (along with the total charge for that peak); each protein gives rise to a charge-state distribution of 4–10 identifiable peaks. (Upper) Intact 70S ribosomes. (Lower) 50S subunits. The spectra are highly reproducible; ca. 100 experiments with 16 separately prepared solutions of ribosomes yielded essentially identical spectra. Control spectra of acid denatured ribosomes show no peaks, confirming that only intact ribosomes are retained during the buffer exchange and that the proteins observed in the mass spectra dissociate from the ribosomes after introduction into the mass spectrometer.