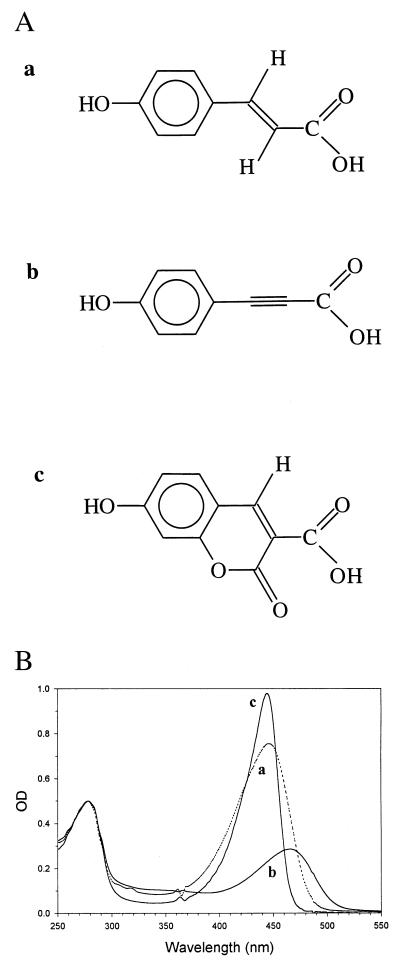
Figure 1.
Chemical structure of the chromophores used for the reconstitution of PYP in this investigation and the absorption spectra of holoPYP and the two hybrids. (A) Chemical structure of 4-hydroxycinnamic acid (a), 4-hydroxyphenylpropiolic acid (b), and 7-hydroxycoumarin-3-carboxylic acid (c). (B) Room temperature UV/Vis absorption spectra of apoPYP, reconstituted with the three chromophores shown in A, resulting in (holo)PYP (trace a), hybrid I (trace b), and hybrid II (trace c). The spectrum of hybrid I was recorded after equilibration in the dark for 30 min. The protein concentration in each sample was approximately 10 μM. The three spectra have been normalized to A = 0.5 at 278 nm. The structure of 4-hydroxycinnamic acid and the spectrum of holoPYP are shown for comparison. The irregularities in these spectra at 363 and 488 nm are artifacts, caused by the diode array spectrophotometer.