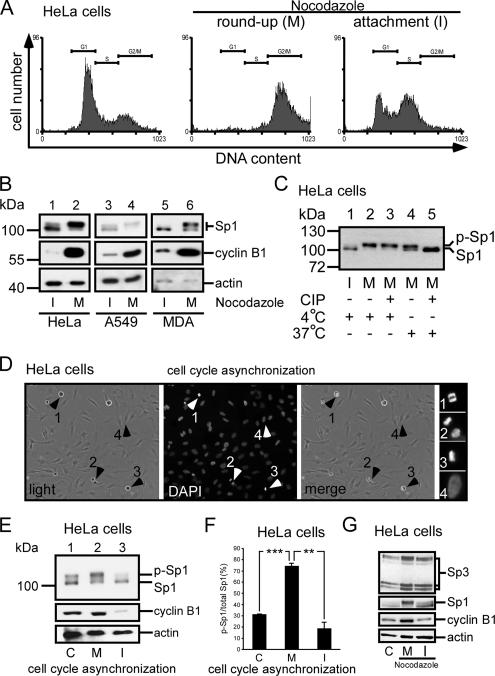
Figure 2.
Sp1 was highly phosphorylated during the mitotic stage. HeLa cells were treated with 45 ng/ml nocodazole for 16 h, and then they were divided into two parts: round-up and attachment cells. To check the stage of the cell cycle, samples were taken for FACS and Western blot analysis. (A) FACS results of paraformaldehyde-fixed, propidium iodide-stained cells are shown as histograms, and the positions of the G1 phase peak, S phase, and G2/M phase peak are marked by horizontal lines. For comparison, untreated HeLa cells were used (left). (B) Immunoblots of cellular extracts of HeLa, A549 and MDA-MB-231 cells in attachment (interphase, I) and round-up (mitotic stage, M) cells were probed using anti-Sp1 antibodies. Cyclin B1 detected by using anti-cyclin B1 antibodies was used as M phase marker, and actin was used as an equal loading control. (C) Mitotic cell extracts were treated with alkaline phosphatase (CIP) to dephosphorylate Sp1, and incubated at 37°C. For comparison, alkaline phosphatase at 4°C has no enzyme activity to dephosphorylate Sp1. The samples were then analyzed using immunoblotting with anti-Sp1 antibodies. (D) In the normal cultured HeLa cells, the round-up and attached cells were separated, and the cell cycle stage was then checked by DAPI. The arrows 1–3 represent that cells stayed in mitosis stage, and arrow 4 represents that cells stayed in interphase. (E). Equal cell number from mixture (control total cells, lane 1), mitosis (round-up cells, lane 2) and interphase (attachment cells, lane 3) stages was used to detect the Sp1 phosphorylated level by using immunoblot of anti-Sp1 antibodies. (F) After three independent experiments, the level of Sp1 phosphorylation was quantified. Only statistically significant p values are shown (**p < 0.01 and ***p < 0.001). (G) Cells in interphase and mitotic stages synchronized by nocodazole (lanes 2 and 3) or control HeLa cells (lane 1) were pooled, and the cellular extracts were then probed using anti-Sp3 antibodies. Sp1 detected by using anti-Sp1 antibodies was used as a positive control, cyclin B1 as an M phase marker and actin as an equal loading control.