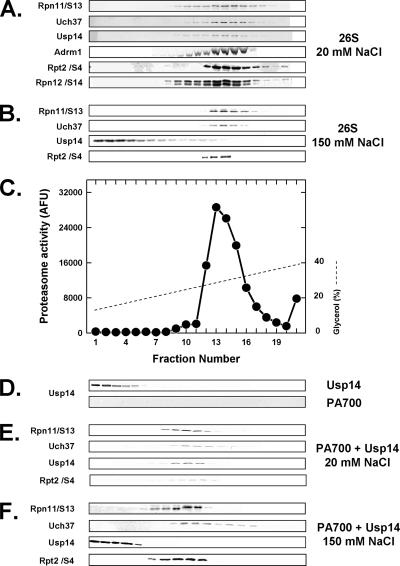
Figure 2.
26S proteasome contains two constitutive and one salt-dissociable deubiquitylating proteins. Purified 26S proteasome (50 μg; A–C), purified PA700 (50 μg; D, bottom, E, and F), and purified recombinant Usp14 (10 μg; D, top) were subjected to glycerol density gradient centrifugation in buffer containing 20 mM Tris-HCl, pH 7.6, 1 mM β-mercaptoethanol, 1 mM ATP, and 5 mM Mg2+ and either 20 mM NaCl (A, C, D, and E) or 150 mM NaCl (B and F). Purified recombinant Usp14 (5 μg) was preincubated with PA700 for 15 min at 25°C in the presence of 20 mM NaCl (E) or 150 mM NaCl (F) before centrifugation. Gradient fractions were Western-blotted for the indicated proteins or assayed for proteasome activity using Suc-Leu-Leu-Val-Tyr-AMC substrate.