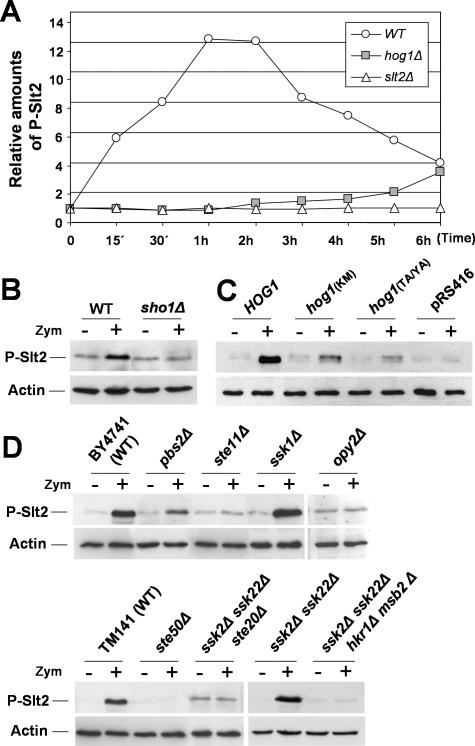
Figure 8.
Elements of the SHO1 branch of the HOG pathway are required for Slt2 activation by zymolyase. (A) Time course of Slt2 activation in hog1Δ cells exposed to zymolyase (0.4 U/ml) for the indicated times compared with the WT and slt2Δ strains. Relative amounts of phosphorylated Slt2 after densitometric analysis, of the Slt2-P are represented. Each data point was first normalized to the total amount of Act1 in the sample and then to the value at time 0 h. (B) Activation of Slt2 after 2 h of treatment with zymolyase is completely lost in a sho1Δ strain. (C) Slt2 activation by zymolyase is lost in the presence of inactivated forms of the Hog1 MAPK. The amount of phosphorylated Slt2 was examined in hog1Δ cells transformed with pRS416 (empty vector), the WT HOG1 allele (pRS416-HOG1), or inactive mutant hog1 alleles (hog1 TA/YA and hog1 KM) after 2 h of zymolyase treatment. (D) Slt2 activation due to zymolyase depends on the presence of SHO1-branch elements of the HOG pathway. The Slt2 phosphorylation status was investigated in WT and strains deleted in different elements of the SHO1 and SLN1 branches: the WT BY4741 and its mutant derivatives pbs2Δ, ste11Δ, ssk1Δ, and opy2Δ, and the WT TM141 and its derivatives ste50Δ, ssk2Δ ssk22Δ, ssk2Δ ssk22Δ ste20Δ, and ssk2Δ ssk22Δ hkr1Δ msb2Δ.