Figure 1.
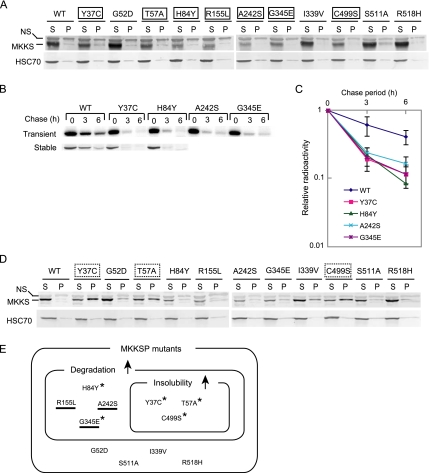
Many disease-causing mutants of MKKS are rapidly degraded and prone to aggregation. (A) HEK293 cells were transiently transfected with 0.3 μg of FLAG-tagged wild-type or mutant MKKS expression vectors. Cell lysates were fractionated by centrifugation (15,000 × g; 15 min), and the resulting supernatant (S) and pellet (P) fractions were analyzed by Western blot analysis. Mutants clearly expressing lower levels than wild-type MKKS are boxed with a solid line. NS, nonspecific band. (B) FLAG-tagged MKKS proteins were transiently expressed in HEK293 cells as described in A (top). Alternatively, HEK293 cells stably expressing wild-type or mutant MKKS were produced (bottom). Cells were radiolabeled for 30 min and chased for 3 and 6 h, and cell lysates were analyzed by immunoprecipitation. (C) The radioactivity of the FLAG-MKKS bands (mean and SD) was determined from three transient expression experiments. (D) HEK293 cells were transiently transfected with 0.5 μg of FLAG-tagged wild-type or mutant MKKS expression vectors and analyzed as described in A. Mutants showing greatly enhanced insolubility (P/S ratio equal to or lager than 1.0) are boxed with a dotted line. (E) Classification of disease-causing MKKS mutants according to their enhanced degradation and insolubility characteristics. The upward arrows denote enhanced characteristics relative to the wild-type protein. Mutants failing to localize to the centrosome are underlined (Figure 3). Mutants exhibiting increased insolubility in the presence of MG-132 (Figure 4B) are indicated by asterisks.