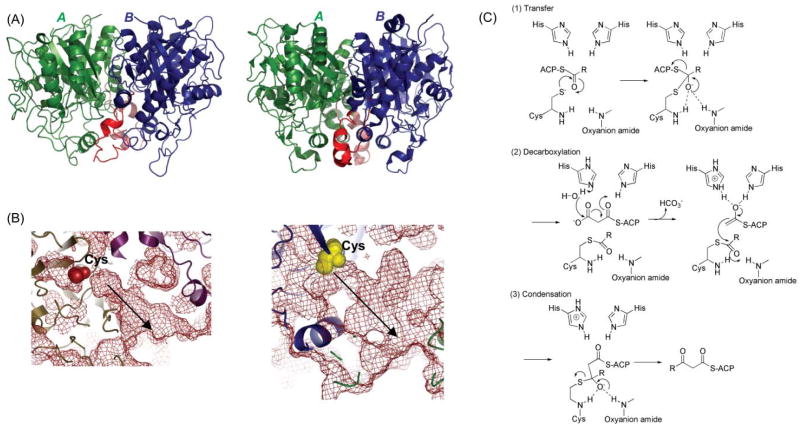
Fig. 18.
The KS domain. (A) Overall fold comparison: left, DEBS KS; right, FAS KS; monomers are shown in green and blue and the dimer interface helix-loop in red. (B) Molecular basis of substrate specificity. The FAS KS channel is narrower with limited flexibility, reflecting its limited substrate tolerance for branched acyl chains, while the DEBS KS5 channel is much wider, reflecting its substrate tolerance; the substrate-binding pockets are identified by arrows. The active site Cys is shown as yellow or red spheres in both panels. (C) Proposed mechanism.