FIGURE 2. PKC is deactivated by Wnt5A inhibition.
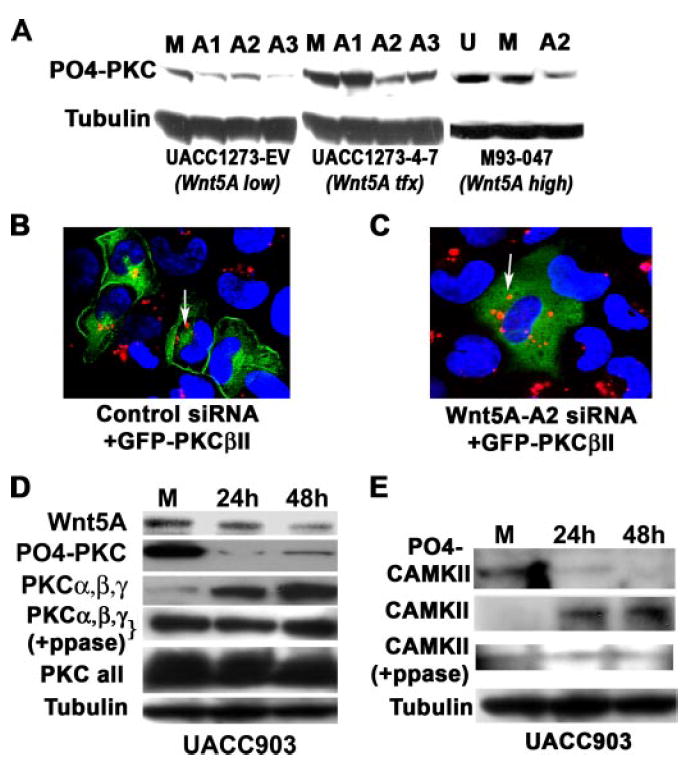
A, the knockdown of WNT5A also results in the dephosphorylation of PKC, indicating a loss of activity. The sequence A2 was the most effective at decreasing PO4-PKC in the Wnt5A stably transfected cells (UACC1273 4-) and is also extremely effective the M93-047 endogenous Wnt5A-expressing cells, without significantly affecting PO4-PKC in the empty vector control cells (UACC1273-EV). B, siRNA against WNT5A deactivates PKC, causing a translocation from the membrane to the cytoplasm. Melanoma cells with high Wnt5A were transfected with GFP-tagged PKC and a control rhodamine-tagged siRNA. In these cells, the PKC is largely membrane-bound as indicated by confocal microscopy (left panel). C, when these cells are co-transfected with GFP-PKC and rhodamine-tagged WNT5A siRNA, the PKC moves away from the membrane and into the cytoplasm (right panel), indicating its de-activation, presumably as a result the observed de-phosphorylation of PKC. D, WNT5A siRNA decreases phospho-PKC (PO4-PKC), but increases non-phosphorylated PKCα, -β, and -γ overtime (PKCα,β,γ). Phosphatase treatment unmasks epitopes that antibodies against total PKC PKCα, -β, and -γ, cannot otherwise recognize, indicating Wnt5A does not have significant effects on the total pool of PKCα, -β, and -γ (PKCα,β,γ +PPase). When using antibodies to all the isoforms of PKC, levels appear to be equal, because Wnt5A does not affect all isoforms, and many migrate at the same size on a gel. E, knockdown of WNT5A using siRNA also results in a decrease in PO4-CAMKII (active), with an increase in non-phosphorylated CAMKII (inactive). Although not as definitive as the PKCα, -β, and -γ data, due to high background on the Westerns, post-phosphatase treatment, the same may also be true of antibody against CAMKII (CAMKII +PPase).
