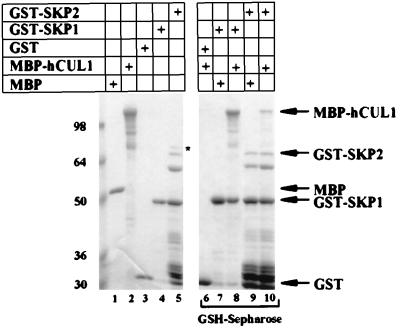
Figure 3.
Human CUL1 binds directly to hSKP1 and SKP2. MBP, MBP-hCUL1, GST, GST-hSKP1, and GST-SKP2 were expressed individually in and purified from bacteria. Each protein was present in the binding reactions at 65 μg/ml. Proteins (4 μg of each) were loaded in lanes 1–5, which represents 1/5 of the input for the binding reactions. Proteins were mixed as indicated (lanes 6–10) and incubated on ice for 1 hr. GST and GST fusions were collected on glutathione-Sepharose (GSH-Sepharose) for 1 hr at 4°C, and the beads then were washed three times with 20 mM Hepes (pH 7.6), 150 mM NaCl, 0.1% Triton X-100, 5 mM MgCl2, 5 mM EDTA, and 2 mM DTT. Proteins bound to glutathione-Sepharose were resolved by SDS/PAGE and were visualized by staining with Coomassie blue. Positions of the full-length fusion proteins are indicated by the arrows. An ≈70-kDa band that copurified with GST-SKP2 from bacteria is marked by an asterisk.