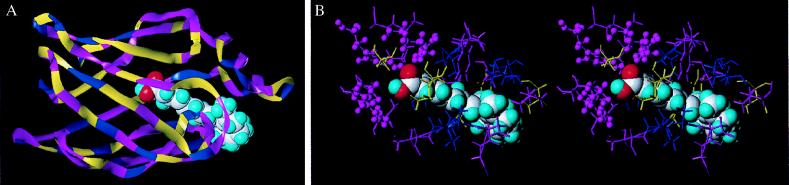
Figure 5.
(A) 3-D structure of msCRABP generated by homology model building using the b/m CRABP I crystal structure as a template. Residue identities, conservative substitutions, and differences between the b/m and Manduca protein are colored magenta, blue, and yellow, respectively. Bound RA is colored gray and turquoise, and red denotes the oxygen atoms of the carboxyl group, which interact with the P2 motif. (B) Stereo molecular model of the msCRABP putative RA binding pocket. The color coding demonstrates the high degree of similarity between msCRABP and b/m CRABP I binding pockets. Identities, conservative substitutions, and differences are in magenta, blue, and yellow, respectively. The oxygen atoms (red) of RA’s (gray and turquoise) carboxyl group interact with the P2 motif (R107, R127, and Y129; magenta “stick and ball” models).