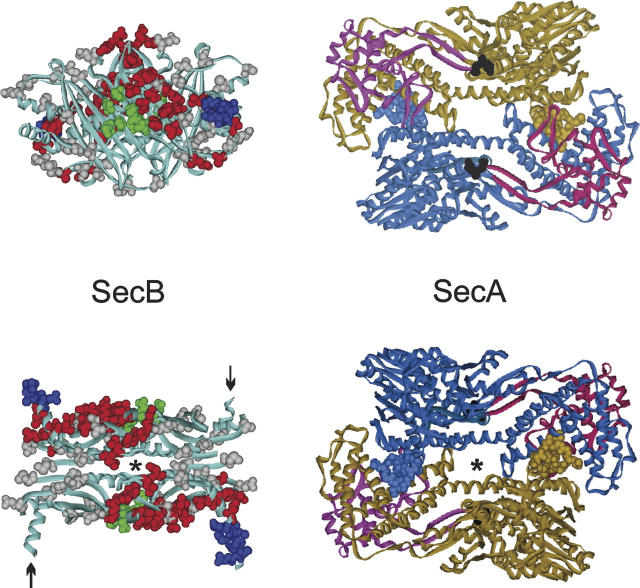
Figure 1.
Docking between SecB and SecA. (Left) SecB X-ray crystal structure. The structure in the lower panel is related to the view in the upper panel by a rotation of 90° toward the viewer around the horizontal axis. The residues shown on the structure as CPK models are those analyzed by site-directed spin labeling or shown to be contacts by X-ray crystallography as described in the text. The positions that showed changes in spectral line shape in the spin label study when SecA was added are displayed in red or blue; those that showed no change, in gray. The contacts defined by X-ray crystallography are shown in green. In the lower panel, the C-terminal α-helices shown in blue CPK models emerge from the upper face, while those indicated by arrows emerge from the lower face. The structure is that of Haemophilus influenzae because the C-terminal residues are resolved (Xu et al. 2000). (Right) SecA X-ray crystal structure from Bacillus subtilis (Hunt et al. 2002). The structure in the upper panel has the PPXD (red and magenta) on the upper surface. The structure in the lower panel is related to the upper panel by a rotation of 180° around the horizontal axis and has the PPXD on the lower surface. The C-terminal zinc-domain of SecA was not resolved in the crystal structure but would emerge from the residues displayed as black CPK models. Amino acid residues 1–11 are shown for each monomer as blue and brown CPK models. The axes of symmetry for both SecB and SecA are indicated (*). The views in the lower panel are positioned to facilitate envisioning docking. The SecA would slide over the top of SecB with the symbol indicating the axes of symmetry aligned. The PDB codes are 1M74, SecA; 1FX3, SecB.