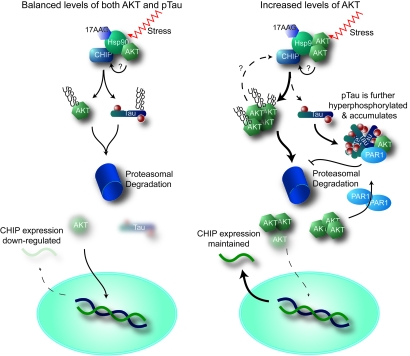
Fig. 6.
Proposed mechanism by which unconventional Akt activity affects tau degradation and CHIP regulation. (Left) Under normal conditions, stress or Hsp90 inhibition (17-AAG) stimulates the CHIP/Hsp90 complex to recognize both Akt and phospho-tau, targeting them for proteasome-mediated degradation by ubiquitination. A consequence of reduced Akt levels would lead to decreased CHIP levels, either through direct transcriptional regulation or via a feedback mechanism. Akt may also regulate the CHIP/Hsp90 complex directly. (Right) As Akt levels increase with age or disease, the CHIP/Hsp90 complex either targets Akt over phospho-tau or alters CHIP activity. As tau accumulates, Akt and PAR1 synergize to enhance tau phosphorylation. Increased levels of Akt maintain CHIP expression.