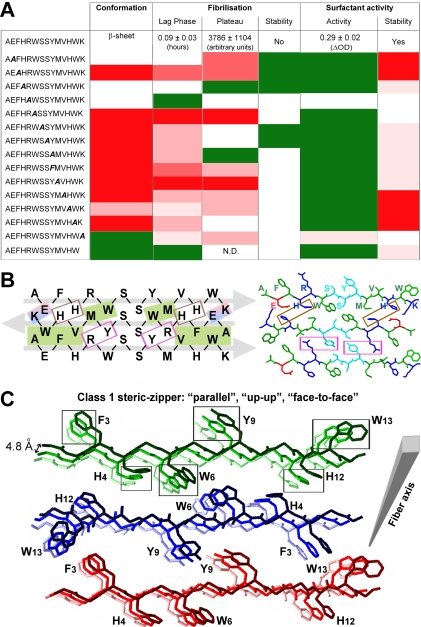
Figure 10. Structural model for AChE586-599 amyloid assembly based on conformation, fibrilization and surfactant properties of the wild type and mutant peptides.
(A) Summary of the conformation, fibrilization and surfactant properties of AChE586-599 and mutant peptides. Mutant peptides with similar properties to AChE586-599 (white boxes), with enhanced properties (green boxes), with diminished properties (red boxes, with low decrease indicated by light red and strong decrease by dark red). ‘N.D.’ indicates ‘non-detectable’. (B) Model of interactions between residues of AChE586-599 β-strands, which form a steric-zipper interface between the fibril forming β-sheets. The steric-zipper interface is shown as an antiparallel assembly of three AChE586-599 β-strands. AChE586-599 is represented at the primary amino acid sequence level (left panel) or at the carbon backbone structure level (right panel). On the left panel, hydrophobic interactions are represented as green shaded boxes, electrostatic interactions as blue and red shaded boxes, cation-π interactions as pink boxes and potential metal binding sites as brown boxes. The grey arrows indicate the direction of the strands. On the right panel, the chains are colored by residue type with hydrophobic residues (A, F, W, M and V) in green, negatively charged (E) in red, positively charged (H, R and K) in blue, and polar (S and Y) in cyan. (C) Model of quaternary interactions within β-sheets of AChE586-599 within a fibril. Each β-sheet is represented with only 3 copies of AChE586-599 for clarity: sheet 1 colored in different shades of green, sheet 2 in different shades of blue, and sheet 3 in different shades of red. The fibril is growing from the lighter to the darker color. On the carbon backbone structure, only the side chains of aromatic residues (F3, H4, W6, Y9, H12 and W13) are represented for clarity. The boxes highlight possible aromatic interactions (π-π) between strands within a β-sheet. Within a β-sheet, AChE586-599 strands are stacking in a parallel arrangement. Within AChE586-599 fibril, the β-sheets are antiparallel, have the same sides facing each other (‘face-to-face’) and the orientation of the sheet edges facing up (‘up-up’). According to the nomenclature of Sawaya et al., this type of arrangement and orientation corresponds to a class 1 steric-zipper [70].