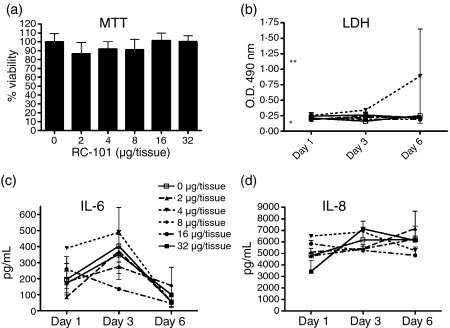
Figure 4.
RC-101 does not alter the viability or inflammatory profiles of human vaginal tissue. Full-thickness vaginal tissues were treated with 100 μl PBS (control) or PBS containing 2–32 μg/tissue RC-101 on day 0 and then washed and re-applied with 50 μl PBS or PBS containing half-doses of RC-101 (1–16 μg/tissue, respectively) at days 1 and 3 postinfection. Tissue viability was assessed by measuring MTT reduction at day 6 post-treatment (a) and LDH activity in the underlay media collected on days 1, 3 and 6 post-treatment (b) Media alone (negative control) and LDH-spiked media (positive control) values are indicated by a single and double asterisk, respectively (b). Underlay media collected on days 1, 3 and 6 post-treatment were also assayed for IL-6 (c) and IL-8 (d) by ELISA. Data are presented as mean ± SEM, n = 4.