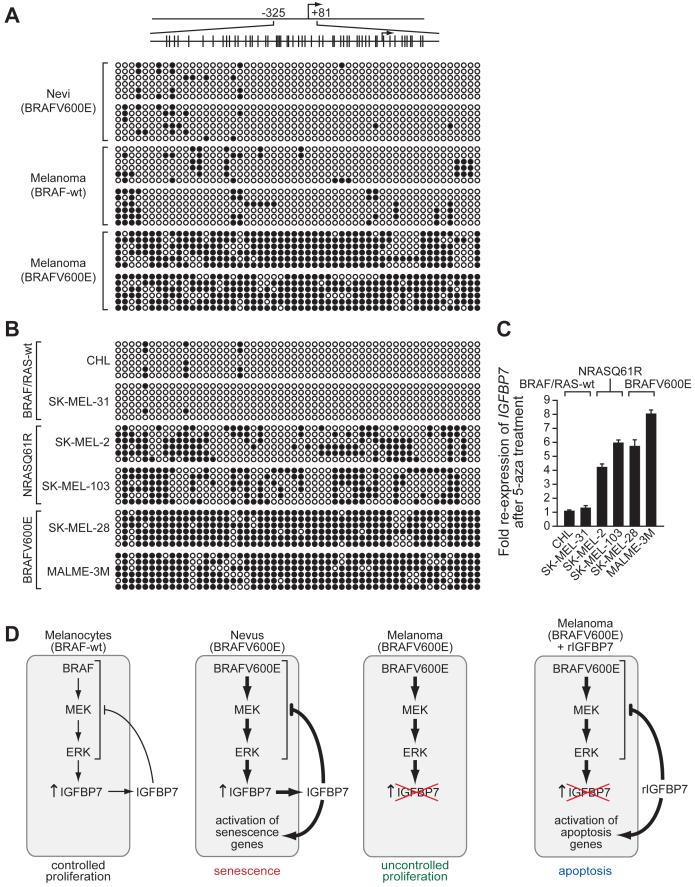
Figure 7. The IGFBP7 Promoter is Hypermethylated in BRAFV600E-Positive Melanoma Cell Lines and Tissues.
(A) Bisulfite sequence analysis of the IGFBP7 promoter in human tissue samples. (Top) Schematic of the IGFBP7 promoter; positions of the CpG dinucleotides are shown to scale by vertical lines. (Bottom) Each circle represents a CpG dinucleotide: open (white) circles denote unmethylated CpG sites and filled (black) circles indicate methylated CpG sites. Each row represents a single clone.
(B) Bisulfite sequence analysis of the IGFBP7 promoter in a panel of melanoma cell lines.
(C) qRT-PCR analysis of IGFBP7 mRNA levels in melanoma cell lines following treatment with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (5-aza). Error bars represent standard error.
(D) Schematic summary of BRAFV600E-mediated senescence and melanoma progression. Normal melanocytes (BRAF-wt) express and secrete low levels of IGFBP7, which inhibits BRAF-MEK-ERK signaling through an autocrine/paracrine pathway, thereby restraining proliferation. In BRAFV600E-positive nevi, constitutive activation of the BRAF-MEK-ERK pathway increases expression and secretion of IGFBP7, and the resultant high levels of IGFBP7 inhibit BRAF-MEK-ERK signaling and activate senescence. In a BRAFV600E-positive melanoma, IGFBP7 expression is lost, enabling the cells to escape from senescence and resulting in uncontrolled proliferation. Addition of exogenous IGFBP7 to BRAFV600E-positive melanoma cells inhibits BRAF-MEK-ERK signaling and activates apoptosis.