Figure 1.
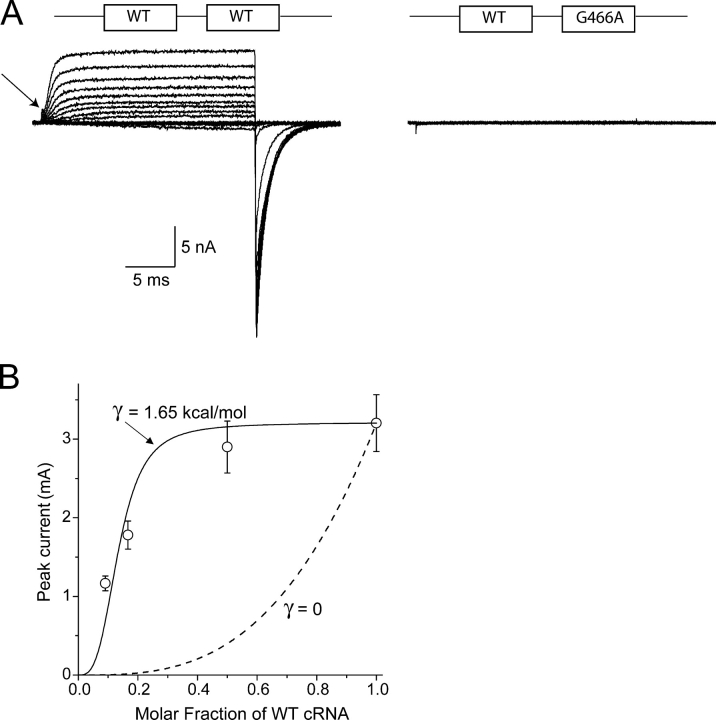
Wild-type subunits cannot rescue the lethality of the G466A subunits. (A) Whole cell Cs+ currents in tandem dimers. Holding potential, −120 mV, depolarizations from −80 to +60 mV in 10-mV increments. WT-WT tandem produces currents comparable to those of WT monomers. The arrow shows gating current. A tandem dimer of WT and G466A, with the mutant downstream of the WT, is completely nonfunctional. Cotransfected tsA201 cells were identified by 4-μm beads coated with CD8 antibody (Dynal Biotech). The tandem construct included the extra linker residues -NNNNNNAMN- between the two protomers. (B) Dominant-negative suppression. Coexpression of WT and G466A monomers reduces peak WT current amplitude at +70 mV in oocytes (n = 15–18 oocytes for each data point). The same molar amount of cRNA for WT was injected in each condition, with the remaining volume adjusted appropriately with water or mutant cRNA. The abscissa represents the molar fraction of WT cRNA. The dashed line is the suppression predicted by the binomial equation. The solid line is a single-parameter fit of a model in which there is an assembly penalty of 1.65 ± 0.14 kcal/mol for each mutant–WT contact in a tetramer (APPENDIX).