Figure 6.
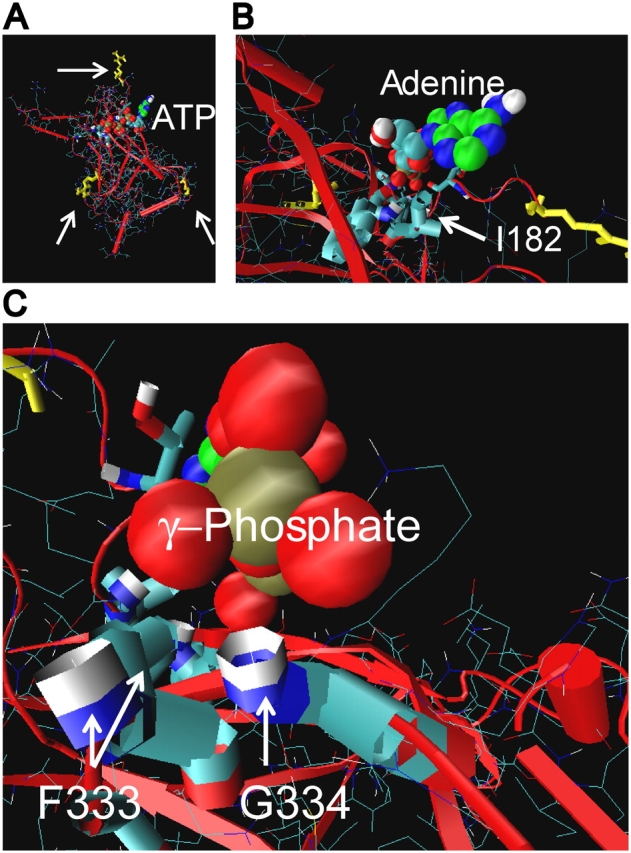
Relationship between membrane, ATP site groove, and cytoplasmic COOH-terminal domain of Kir6.2. (A) Structural model of cytoplasmic COOH-terminal domain of Kir6.2. Arrow at top points to R177, which is thought to interact with the inner leaflet of the membrane. Arrow at lower left points to R314, and at right points to E229, which form ion pairs between adjacent subunit domains (Lin et al., 2003). Downward from R177, I182 is at the right end of the putative ATP site groove and G334 is at the left end. A favorable crystallographic ATP conformer complexed onto the Kir6.2/3.1 homology structure by AUTODOCK is shown. The triphosphate (red oxygen and brown phosphorus balls) extends leftward along the groove. The adenine (green carbon and blue nitrogen balls) extends up and out of the groove. The binding site groove, therefore, appears to be relatively high on the cytoplasmic domain, and facing out to the cytoplasm toward the inner leaflet of the membrane. (B) Close-up view of the adenosine moiety binding near the hydrophobic side chain and hydrophilic peptide bond amide of I182. (C) Close-up view of the oxygens of the γ-phosphate of ATP approaching the backbone amides of F333 and G334, where favorable hydrogen bonding could occur. The proximity of the two amides and γ-phosphate of ATP, together with the opposite effects of positive-charged side-chain substitutions at 333 and 334 versus ATP occupancy of the site, suggests an electrostatic environment of this end of the ATP site that can alter gating at the transmembrane domain.
